Halicephalobus Timm, 1956
|
publication ID |
https://doi.org/ 10.1080/00222933.2021.2006352 |
|
DOI |
https://doi.org/10.5281/zenodo.6204758 |
|
persistent identifier |
https://treatment.plazi.org/id/5E2C6265-FFC9-FFE0-FF3A-FF3282E483C2 |
|
treatment provided by |
Plazi |
|
scientific name |
Halicephalobus Timm, 1956 |
| status |
|
On the distribution and association of the genus Halicephalobus Timm, 1956 View in CoL
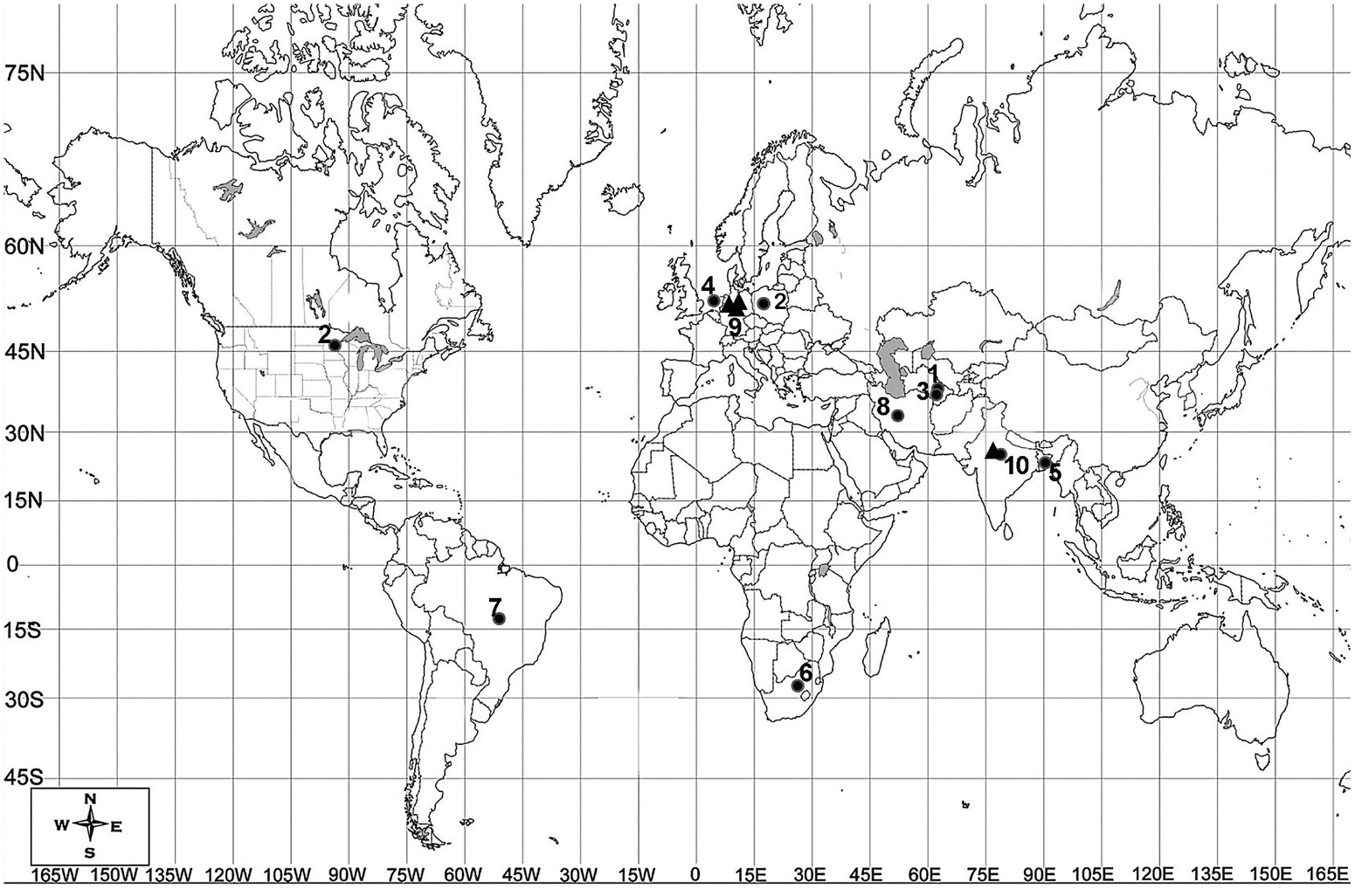
Halicephalobus is largely a cosmopolitan bacteriophagous genus, which is known for its diverse habitat range. The original localities of representative species show a diversity of geographical ranges around the globe ( Figure 8 View Figure 8 ). The species have been predominantly reported from soil, rotten wood and bark, sap flux, tree holes, tree trunks, dung piles etc., indicating greater chances of their association with insects or other opportunistic, r-selective invertebrates. The species H. gingivalis has been found to be distributed worldwide, with records from Colombia, Egypt, Japan, the United Kingdom, the United States, Switzerland and The Netherlands. They show the ability to survive in hostile environments (i.e. anoxic conditions) or conditions of desiccation and dehydration. In some instances the probability of their survival in harsh conditions has been demonstrated; for example, H. laticauda was found 630 m below sea level in the water pit of a coal mine ( Geraert et al. 1988), while H. mephisto was found 1.3 km deep in Beatrix gold mine, South Africa ( Borgonie et al. 2011).
The presence of caudal glands and a probable sclerotised spinneret may be an adaptation of H. laticauda in an aquatic environment to cling to the substrate. The structure seems to be faintly visible in H. termitis and H. similigaster , probably indicating their role in phoresy. A few species also show various types of associations with invertebrates and vertebrates ranging from phoresy to pathogenesis. Halicephalobus gingivalis ( Stefanski, 1954) Andrássy, 1984 serves as a pathogen of horses and humans and has been found in the nostrils of horses causing ataxia, granulomatous inflammation and destruction of infected tissues of various organs along with renal dysfunction ( Gardiner et al. 1981; Blunden et al. 1987; Anderson et al. 1998; Nadler et al. 2003).
In the present study, out of 15 colonies of termites, 10 yielded species of Halicephalobus . Infective juveniles largely invaded the head of termites or, in rare instances, the abdominal cavity. Earlier, populations of Halicephalobus sp. were reportedly isolated ( Kanzaki et al. 2011) from the colonies of different species of termites (e.g. Neotermes koshunensis and Cryptotermes domesticus of the family Kalotermitidae ; Coptotermes formosanus of Rhinotermitidae ; Odontotermes formosanus and Nasutitermes takasagoensis of Termitidae ). The association of Halicephalobus sp. with Rhinotermes lucifugus (Rhinotermitidae) was also reported by Fürst Von Lieven and Sudhaus (2008).
Another conspicuous feature of the species of Halicephalobus is their parthenogenetic lineages. In sexually reproducing amphimictic species, variations lead to better adaptable generations. However, parthenogenetic or non-sexually reproducing species show lesser chances of divergence. Nevertheless, their diversification into different niches, geographical isolation and the resulting divergent adaptations may be the key to speciation and species diversification ( Barraclough and Herniou 2003).
The following key can help in the identification of species of Halicephalobus .
1. Tail only 2 anal body diameters.................................................................................. brevicauda
– Tail more than 2.5 anal body diameters..................................................................................... 2
2. Tail with caudal gland remnants .................................................................................................. 3
– Tail without caudal gland remnants ........................................................................................... 5
3. Tail with sclerotised string at the terminus ............................................................. laticauda
– Tail without or with inconspicuous sclerotisation ................................................................. 4
4. Vulva far posterior (V = 71–75)........................................................................................... termitis
– Vulva equatorial to slightly post equatorial (V = 49–68) ................................ similigaster
5. Stoma with metastegostomal denticle ...................................................................... gingivalis
– Stoma without discernible denticle ............................................................................................ 6
6. Eggs>50 um long, stoma 8–10 times longer than wide .......................................... limuli View in CoL
– Eggs up to 45 um long, stoma 4–6 times longer than wide ............................................ 7
7. Lip region flattened, stoma relatively wider ............................................................................ 8
– Lip region rounded to conical, stoma narrower .................................................................... 9
8. Ovary without flexure; tail filiform ............................................................................... mephisto
– Ovary with flexure, tail long conoid ............................................................................... persicus View in CoL
9. Lip region narrower than adjoining body, tail almost straight ......................... palmaris
– Lip region as wide as the adjoining body, tail ventrally curved ................ intermedius
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |