Tellervotrema katadara ( Kuramochi, 2001 ) Kuramochi, 2009
|
publication ID |
https://doi.org/10.11646/zootaxa.3986.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:B84A49B3-F5F3-44AF-B270-038D6D28A4A2 |
|
DOI |
https://doi.org/10.5281/zenodo.5670166 |
|
persistent identifier |
https://treatment.plazi.org/id/5D5A5F74-0E78-FFC4-B3AD-805F6DEA698C |
|
treatment provided by |
Plazi |
|
scientific name |
Tellervotrema katadara ( Kuramochi, 2001 ) Kuramochi, 2009 |
| status |
|
Tellervotrema katadara ( Kuramochi, 2001) Kuramochi, 2009 View in CoL
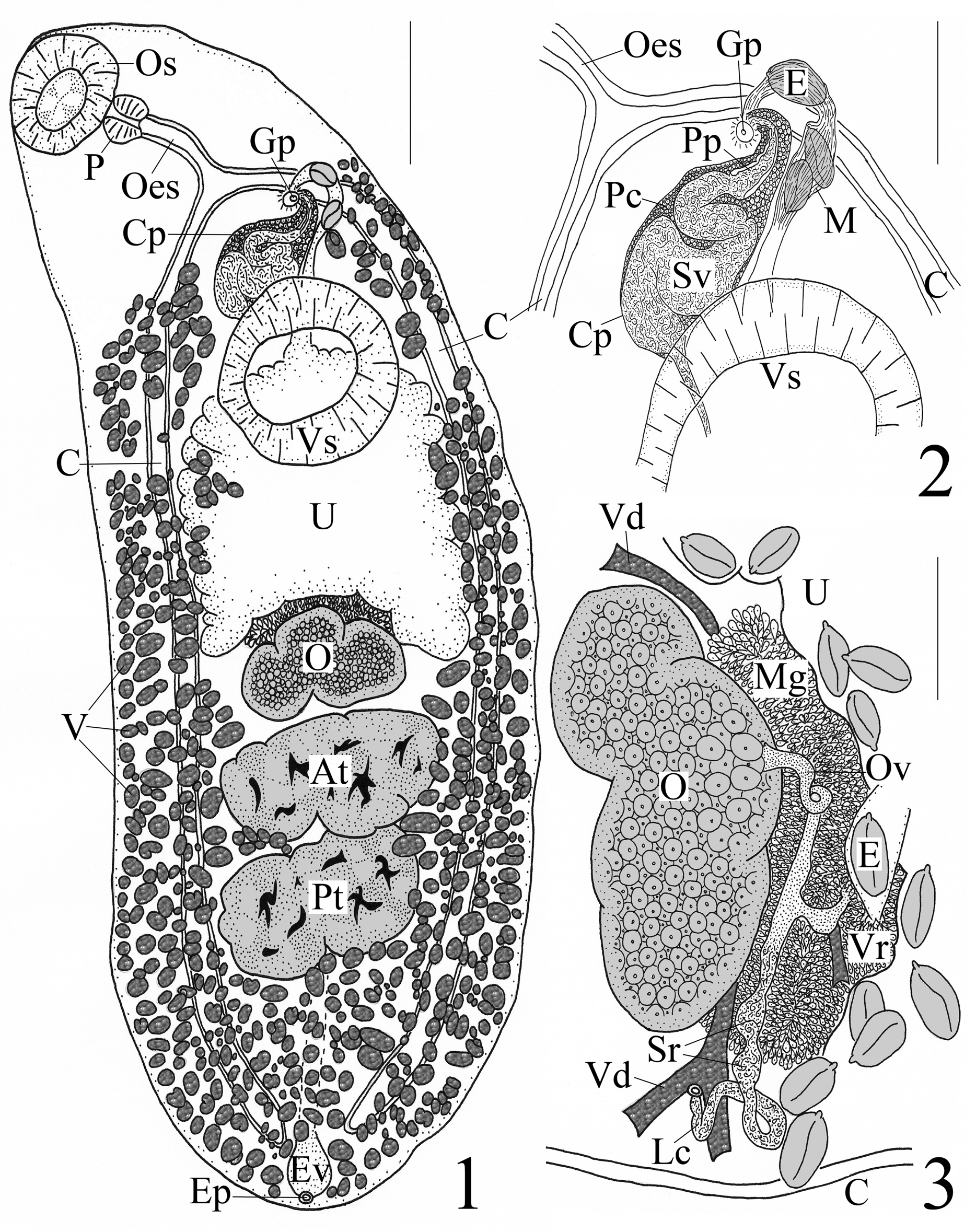
( Figs. 1–3 View FIGURES 1 – 3 )
Synonym. Plagioporus katadara Kuramochi, 2001
Type-host. Gadomus colletti Jordan & Gilbert; Gadiformes : Macrouridae : Bathygadinae .
Type-locality. Tosa Bay, off the Pacific coast of southern Japan, 33°11.09’N, 133°40.11’E – 33°11.91’N, 133°40.99’E, depth = 518–522 m, 25/June/2000.
Other locality. Tosa Bay, off the Pacific coast of southern Japan, 33°08.517’N, 133°38.557’E – 33°07.739’N, 133°37.829’E, depth = 577–582 m, 17/June/1999.
Site. Intestine.
Deposited Specimens. Collector TK, holotype NSMT-Pl 5143ab, 5 paratypes NSMT-Pl 5110 & NSMT-Pl 5143ab.
Records. 1. Kuramochi (2001); 2. Present study.
Descriptions. 1, 2.
Re-description. [Based on 1 holotype and 5 paratypes. Measurements and proportions given in Table 2.] Body elongate-oval, widest equatorially; rounded at both ends. Forebody attenuated anteriorly, narrows at oesophageal level. Hindbody rounded posteriorly, wider than forebody, with parallel margins, narrows in post-testicular region. Tegument smooth. Pre-oral lobe absent. Oral sucker subspherical, subterminal. Ventral sucker sessile, round to transversely elongate, wider than long in most specimens, larger than oral sucker, post-bifurcal, pre-equatorial and located near junction of anterior and middle thirds of body. Prepharynx short in 67% [n=4] of specimens and indistinct in 33% [n=2] of specimens. Pharynx muscular, dolioform, wider than long in 50% [n=3] of specimens. Oesophagus thick-walled, straight to somewhat sinuous. Intestinal bifurcation anterior to ventral sucker by holotype 208 (mean 194, range 104–424) [n=5] long. Ceca narrow, run posteriorly along lateral sides of worm, end blindly near posterior extremity; cecal ends arcuate.
Parasite T. katadara T. katadara T. katadara n = 1 4 1
Taxonomic status Holotype Paratypes Paratype Source of data NSMT-Pl 5143ab1 NSMT-Pl 5143ab1 NSMT-Pl 5110 1 Length 2,640 1,640–1,740 (1,690)2 4,320 Width at pharynx 536 360–560 (452) 820
Width at VS2 928 576–864 (702) 1,360 Width at PT2 1,000 640–896 (740) 1,280 Forebody L2 740 540–580 (560) 1,340 Hindbody L 1,520 840–900 (857) 2,500 Oral sucker (OS) L 240 184–208 (196) 328
OS W2 288 220–288 (244) 352
Prepharynx L 0 0–20 (15) 48
Pharynx L 100 80–148 (115) 144
Pharynx W 112 84–104 (95) 192
Oesophagus L 220 148–208 (180) 376
VS L 408 268–324 (292) 488
VS W 460 336–424 (370) 464
Post-cecal region L 184 84–124 (99) 280
AT 2 L 248 160–200 (183) 408
AT W 552 376–520 (422) 800
PT L 264 204–240 (223) 432
PT W 520 368–480 (417) 640
AT to PT 0 0 0
Post-testicular region (PTR) L 488 232–280 (254) 584
PTR W at mid-point 760 456–680 (548) 936
Cirrus pouch (CP) L 356 246–500 (335) 792
CP W 152 100–156 (128) 216
CP overlap VS 0 0–152 (65) 208
Ejaculatory duct W in distal CP 10 8–10 (9) [n=3] 12
Genital pore to lateral margin 176 108–148 (130) 224
Pre-ovarian region L 1,420 888–960 (927) 2,660 Ovary (OV) L 232 116–172 (139) 280
OV W 428 264–436 (325) 432
VS to OV 272 80–168 (122) 860
Vitellarium L 68–136 (102) [n = 5]2 32–88 (54) [n=20]2 76–196 (122) [n=5]2 Vitellarium W 52–64 (56) [n=5] 16–48 (31) [n=20] 44–96 (70) [n=5] Vitelline reservoir L 34 72–82 (77) [n=2] Not observed Vitelline reservoir W 20 64–72 (68) [n=2] Not observed Uterus L 960 512–640 (580) 1,840 Uterus W 672 344–624 (421) 860
Post-uterine region (PUR) L 1,140 664–720 (687) 1,600
......continued on the next page 1These specimens were collected and described by TK as Plagioporus katadara Kuramochi, 2001 in Kuramochi (2001, pp. 23–25, Figs. 7–9) and housed in the National Museum of Nature and Science, Tokyo, Japan (NSMT), under these accession numbers.
2AT, anterior testis; L, length; PT, posterior testis; VS, ventral sucker; W, width; range with mean in parentheses; number [n] of measurements if different from total number of worms examined.
3 Proportion of body length.
Testes 2, tandem, smooth to lobed, transversely elongate, contiguous, median, intercecal, post-equatorial mainly in posterior third of body. Post-testicular region occupies posterior fifth of body. Cirrus pouch thin-walled, distinct, clavate, oriented transversely along lateral axis from near midline anterosinistrally to point midway between left body margin and midline proximate to left cecum, post-bifurcal; distal portion pre-acetabular; posterior extent of proximal portion variable and continues to either anterior margin of ventral sucker or extends as far as 1/2 length of ventral sucker. Seminal vesicle voluminous, bipartite; posterior proximal portion wide, saccate, convoluted; anterior distal portion tubular, narrow, passes in nearly straight line anterosinistrally before it turns sharply to right to proceed short distance into small, circular genital atrium. Prostate gland-cells distinct, numerous throughout cirrus pouch with densest number in distal portion of pouch. Pars prostatica conspicuous, thick-walled, nearly straight, 100 (121, 72–200) [n=3] long × 44 (27, 24–28) [n=3] wide, in distal portion of cirrus pouch; ejaculatory duct narrow, distinct, with cirrus present. Genital pore post-bifurcal, pre-acetabular by 180 (205, 124– 416) [n=5] long, submedian, sinistral, midway between left body margin and midline of worm, ventrally overlaps left cecum or located immediately sinistral to left cecum.
Ovary 3- to 4-lobed, median to dextrally submedian, pre-testicular, contiguous with anterior testis, in posterior middle third of body. Mehlis’ gland conspicuous, cells numerous, located anterodorsal to anterior margin of ovary and extends posteriorly to mid-level of ovary in some individuals. Seminal receptacle distinct, canalicular, sinistral to ovary and midline of worm. Laurer's canal opens dorsal and medial to left cecum; canal itself conspicuous, long, thick-walled, runs along anterior margin of ovary, mostly straight until near opening where it loops; lateral half of canal in holotype dilated in 2 places where filled with sperm. Oviduct loops once or twice as it proceeds anteriorly from anterior margin of ovary. Vitellarium circumcecal, follicles numerous, subspherical or oblong or irregular in shape; anterior extent of vitellarium in forebody as 2 separate “bunches” (i.e. Tellervotrema vitellarium distribution) distributed from near level of intestinal bifurcation (one individual with a few follicles on left side slightly anterior to bifurcation) posteriorly either to anterior margin of ventral sucker or to mid-level of latter; posterior extent of vitellarium in hindbody along lateral margins from level of posterior margin of ventral sucker to posterior extremity, not confluent but encroaches over lateral margins of gonads, immediate pre-ovarian region, space between ovary and anterior testis and into inter-testicular area, completely confluent in post-testicular region. Vitelline reservoir small, sinistrally submedian, directly anterior to left lobe of ovary. Vitelline ducts indistinct, pass medially at level near ovary. Uterus intercecal, winds in wide loops to fill up almost entire space between ovary and ventral sucker; proximal loops run anteriorly from mid-level of ovary, narrows and passes dorsal to ventral sucker; distal loop turns anterosinistrally and runs parallel along left side of cirrus pouch before it loops anteriorly to terminate at genital pore. Metraterm thick-walled, mostly straight, runs parallel to left side of cirrus pouch before distal end curves around anterodextrally to enter genital atrium. Eggs oval, moderate in size and number, many collapsed or crenulated, amber, non-filamented, operculate, with conspicuous knob or boss on one pole.
Excretory vesicle tubular, I-shaped, anteriormost extent of vesicle obscure in 50% [n=3] of specimens but observed to extend at least to posterior testis [n=2] and anterior testis [n=1]. Excretory pore dorsal, subterminal.
Remarks. These specimens belong in the Plagioporinae Manter, 1947 because they possess a well-developed cirrus pouch that encloses a seminal vesicle and have a canalicular seminal receptacle. Based on Cribb (2005), these same specimens can be identified as belonging to the genus Tellervotrema by the presence of the following combination of diagnostic characteristics: non-filamented eggs that are> 40 Μm long (ours ranged from 70–82 long); blind ceca; an elliptical, non-pedunculate ventral sucker that lacks lamellar lips, “fleshy folds” or an accessory attachment organ; vitellarium that extend to the posterior end of the body, well into the forebody, and are interrupted at the level of the ventral sucker; tandem and paired testes; an oral sucker that is not funnel-shaped; an excretory vesicle that is not diverticulate and does not extend to the pharynx but terminates inside the hindbody at about the level of the ovary (anterior extent of excretory vesicle was obscure in our specimens, but it extended to anterior testis [cf. about level of ovary] in one individual); a clearly submedian genital pore; a pre-testicular uterus; and our specimens parasitized a macrourid species ( G. colletti ).
As mentioned above, T. katadara was suppressed by Kuramochi (2011). For this study, we examined the specimens of Plagioporus katadara , Tellervotrema katadara and Tellervotrema beringi used by Kuramochi (2001, 2009, 2011). We have concluded that the specimens of “ T. beringi ” documented by Kuramochi (2011) from the pyloric ceca of the short-tailed grenadier, Nezumia proxima , from Sagami Bay, Japan, are in fact a new species of Allopodocotyle Pritchard, 1966 , which we have described in another report (see Blend et al. 2015), and as a result, they will not be considered further in this report. We have also concluded that the specimens of “ T. katadara ” documented by Kuramochi (2009) from the intestine of the longfin grenadier, Coryphaenoides longfilis , from off northern Honshu, Japan, are in reality T. beringi sensu stricto, and we have re-described these specimens below. Thus, it is the holotype and five paratypes of “ Plagioporus katadara ” (NSMT-Pl 5143ab, NSMT-Pl 5110) described by Kuramochi (2001) from the intestine of the bathygadine macrourid Gadomus colletti from Tosa Bay, Japan, that is the present focus herein.
Blend et al. (2012) concluded that six features best distinguished T. armstrongi and T. beringi . Egg size differs for T. beringi (100–110 long × 50–60 wide [see Mamaev 1965] and 80–102 × 40–60 [see Blend et al. 2012]) vs T. armstrongi (50.6–64 × 24–35 [see Gibson & Bray 1982] and 47.5–66 × 24–42 [see Blend et al. 2012]). The position of the genital pore differs for T. beringi (opening at the level of the intestinal bifurcation [bifurcal] but can reach the level of the posterior oesophagus [slightly pre-bifurcal]) vs T. armstrongi (opening at the level of about 30% of the distance between the intestinal bifurcation and the pharynx or more anterior [pre-bifurcal]) (see Gibson & Bray 1982; Blend et al. 2012). The posterior extent of the cirrus pouch relative to the ventral sucker and intestinal bifurcation differs for T. beringi (cirrus pouch terminates well posterior to the level of the intestinal bifurcation often reaching the posterior margin of the ventral sucker) vs T. armstrongi (cirrus pouch terminates at or just posterior to the level of the intestinal bifurcation and is entirely anterior to or only reaches to the middle of the ventral sucker) (see Gibson & Bray 1982; Blend et al. 2012). The testes appear to take up a much larger volume in the hindbody of T. beringi vs T. armstrongi , and the anterior extent of the distinctive, paired, isolated vitelline “bunches” in the forebody of the worm (referred to above as “ Tellervotrema vitellarium distribution”) differs between T. beringi (paired vitelline fields begin noticeably anterior to the intestinal bifurcation) vs T. armstrongi (paired vitelline fields begin at the level of the intestinal bifurcation or a short distance posterior to it) (see Mamaev 1965; Gibson & Bray 1982; Blend et al. 2012). Finally, Blend et al. (2012) noted that both species occupy a different geographic locality; T. beringi resides in the North Pacific Ocean (Bering Sea and off Oregon) while T. armstrongi inhabits the North Atlantic Ocean (off Scotland and in the Gulf of Mexico).
The species “ P. k a t a da r a ” as described by Kuramochi (2001) and re-described above as T. katadara (see Table 2) differs from T. armstrongi and T. beringi in the combination of the six diagnostic characteristics just given. Egg size of P. katadara is 70–82 × 40–52, and this is intermediate in size between T. beringi (larger) and T. armstrongi (smaller). The genital pore of P. katadara is post-bifurcal whereas it is bifurcal in T. beringi and pre-bifurcal in T. armstrongi . In P. katadara , the posterior extent of the cirrus pouch is more like T. armstrongi in that it continues to either the anterior margin of the ventral sucker or extends as far as half the length of it, yet it is more similar to T. beringi in that the cirrus pouch of P. k a t a da r a terminates well posterior to the level of the intestinal bifurcation. The extent of the testes of P. katadara is more like T. beringi in that they take up a larger volume in the hindbody when compared with T. armstrongi (see Fig. 1 View FIGURES 1 – 3 ). The anterior extent of the paired vitelline “bunches” in the forebody of P. katadara is to near the level of the intestinal bifurcation (like T. armstrongi ) and not noticeably anterior to the intestinal bifurcation (like T. beringi ). Lastly, P. k a t a da r a was collected from Tosa Bay, off the Pacific coast of southern Japan; a geographic locality that coincides with T. beringi (N. Pacific Ocean in the Bering Sea and off Oregon) and not with T. armstrongi (N. Atlantic Ocean off Scotland and in the Gulf of Mexico). Thus, P. katadara has certain features that are either completely different (e.g. position of the genital pore), or are intermediate in size (e.g. egg size), or are similar but only in part (e.g. posterior extent of cirrus pouch), when it is contrasted with T. armstrongi and T. beringi . Also evident is the similarity of P. k a t a da r a to T. beringi in testes volume in the hindbody and geographic locality, while P. katadara is similar to T. armstrongi in the anterior extent of the paired vitelline “bunches” characteristic of species of Tellervotrema . Given the unique combination of these differences among the six diagnostic characteristics of Blend et al. (2012), we hereby re-instate Tellervotrema katadara as a recognized species represented by the specimens originally described as Plagioporus katadara by Kuramochi (2001, pp. 23–24, Figs. 7–9); therefore, the total number of accepted species in Tellervotrema is now three.
Our morphometric measurements of this material were close in overall comparison with those of Kuramochi (2001), but we did observe some minor differences. The oral sucker was originally described as terminal; whereas, we found it to be subterminal (see Fig. 1 View FIGURES 1 – 3 ), and this is noticeable as well in Fig. 7 of Kuramochi (2001). The lower limits of the length (160 Μm) and width (210 Μm) of the ventral sucker given by Kuramochi (2001) were less than what we measured for this feature—268 × 336 (see Table 2), yet the sucker width ratios were unchanged. The cirrus pouch was originally described as “at [the] anterior margin of [the] acetabulum”, but we observed the posterior extent of the cirrus pouch to be variable; it continued to either the anterior margin of the ventral sucker or as far as half the length of it. We noted that the single paratype specimen of T. katadara (NSMT-Pl 5110) is a relatively large individual and had features with sizes consistently larger than those given by Kuramochi (2001); however, the holotype and four paratypes of T. katadara (NSMT-Pl 5143ab) had features that were, for the most part, quite similar in size to those in the type description (see Table 2). Finally, Kuramochi (2001) described the seminal receptacle of this species as small and weakly developed, and he illustrated this feature in the holotype (Figs 7 & 9 as “SR”) as a tiny, blind sac located at the junction of the “germiduct” (= oviduct) and the most medial part of the Laurer’s canal. We feel this feature was misinterpreted. We observed the seminal receptacle in the holotype of T. katadara (NSMT-Pl 5143ab) to be two distinct, sperm-filled dilations located sinistral to the ovary and midline of the worm within the lateral (not medial) portion of the Laurer’s canal (see our Fig. 3 View FIGURES 1 – 3 ). What was described and illustrated as the seminal receptacle (“SR”) by Kuramochi (2001) was in reality an extension of the Mehlis’ gland cells and a developing egg behind the Laurer’s canal (original illustrations of holotype were in ventral view). As described above, we did see dilations along the Laurer’s canal proximate to the loop and dorsal opening ( Fig. 3 View FIGURES 1 – 3 ) that appear to be full of fluid (sperm)—the seminal receptacle. Based on the subfamily diagnosis for the Plagioporinae (see Cribb 2005), members of this group have a canalicular seminal receptacle; a seminal receptacle formed by a dilation(s) of the proximal portion of the Laurer’s canal (see Gibson & Bray 1979). Thus, our observation of the sperm-filled dilations along the Laurer’s canal within the holotype specimen of T. katadara is in line with typical members of this subfamily.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Plagioporinae |
|
Genus |