Amaryllidaceae
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2019.112055 |
|
DOI |
https://doi.org/10.5281/zenodo.10581096 |
|
persistent identifier |
https://treatment.plazi.org/id/5C7FA13E-FFF0-6B52-FCF8-9AC0E57DF804 |
|
treatment provided by |
Felipe |
|
scientific name |
Amaryllidaceae |
| status |
|
2.1. Isolation and structural elucidation of Amaryllidaceae View in CoL View at ENA alkaloids
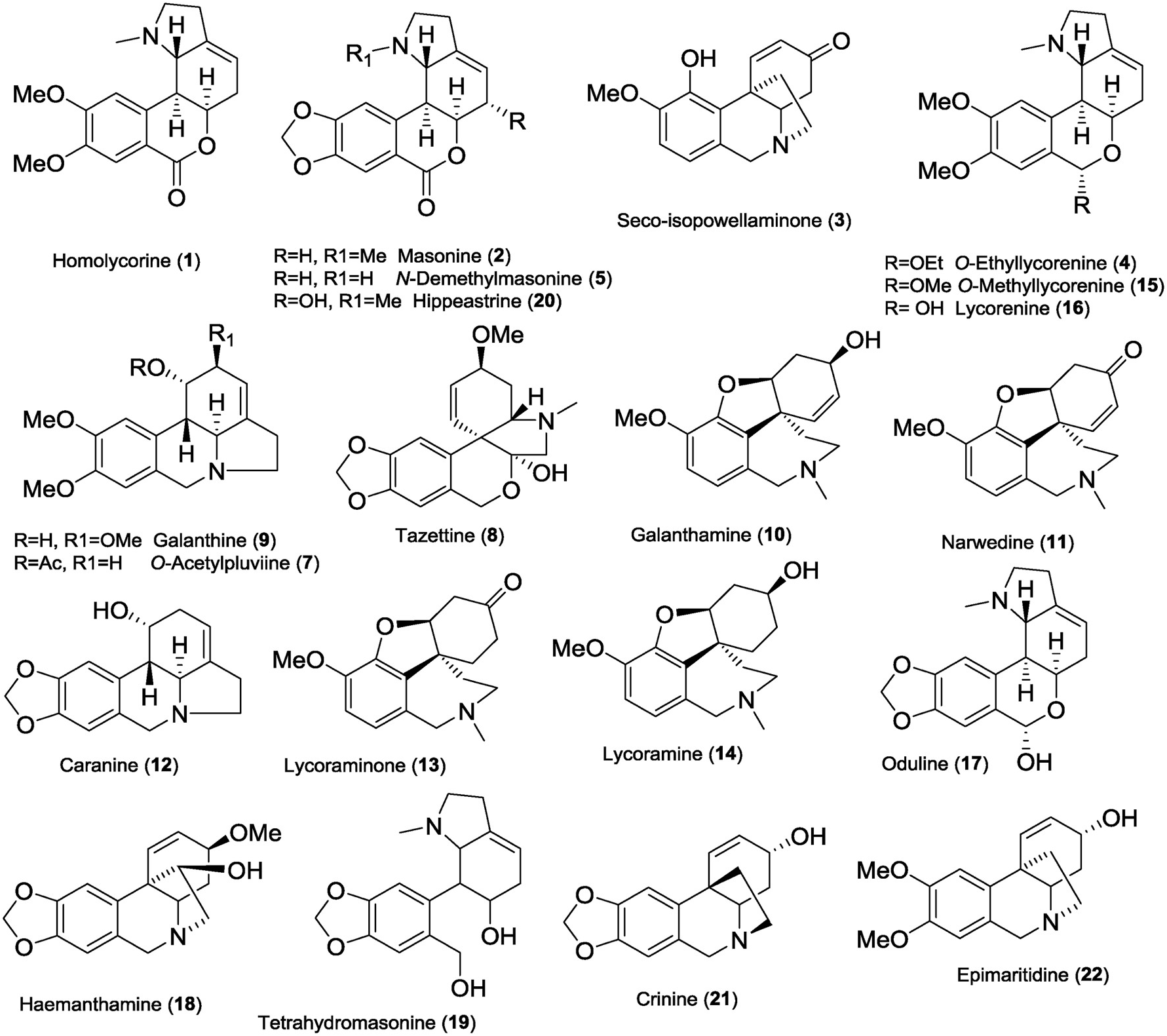
Twenty-one known Amaryllidaceae alkaloids (1–5, 7–22), and one new alkaloid (6) were isolated from fresh bulbs of Narcissus pseudonarcissus cv. Dutch Master ( Amaryllidaceae ) by common chromatographic methods. The compounds were identified by MS, ESI-HRMS, 1D and 2D NMR spectroscopic analyses and by comparison of the obtained data with the literature for homolycorine (1) ( Huang et al., 2013), masonine (2) ( Pigni et al., 2012), seco-isopowellaminone (3) ( Kogure et al., 2011), O -ethyllycorenine (4) ( Huang et al., 2013), N -demethylmasonine (5) ( Kreh and Matusch, 1995), O -acetylpluviine (7) ( Harken et al., 1976), tazettine (8) ( Pham et al., 1999), galanthine (9) ( Kihara et al., 1994), galanthamine (10) ( Chen et al., 2012), narwedine (11) ( Jegorov et al., 2006), caranine (12) ( Lamoral-Theys et al., 2009), lycoraminone (13) ( Lee et al., 1998), lycoramine (14) ( Chen et al., 2012), O -methyllycorenine (15) ( Liu et al., 2015), lycorenine (16) ( Liu et al., 2015), oduline (17) ( Huang et al., 2013), haemanthamine (18) ( Bastida et al., 2006), tetrahydromasonine (19) ( Döpke and Bienert, 1966), hippeastrine (20) ( Jeffs et al., 1985), crinine (21) ( Kobayashi et al., 1984), and epimaritidine (22) ( Ghosal et al., 1985) ( Fig. 1 View Fig ).
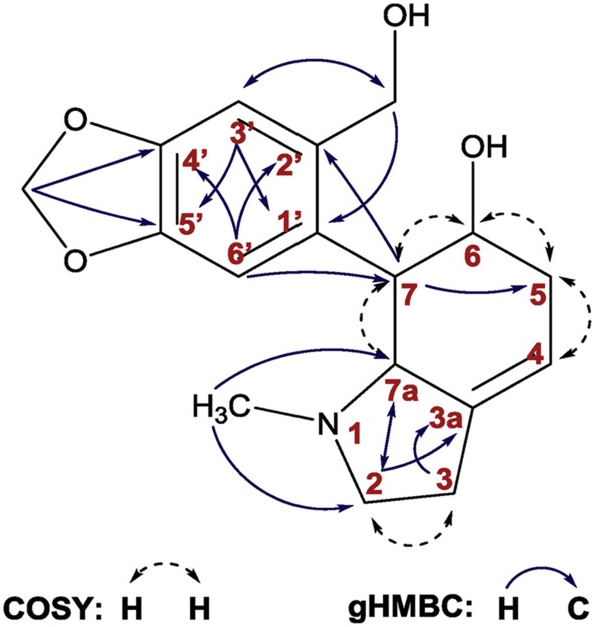
The isolated alkaloids belong to the homolycorine (1, 2, 5, 15–17, 19, 20), galanthamine (10, 11, 13, 14), haemanthamine (3, 18), crinine (21, 22), lycorine (7, 9, 12) and tazettine (8) structure types. The basic core of the novel alkaloid narcimatuline (6) mimics galanthamine and galanthindole, both Amaryllidaceae alkaloids. It is noteworthy that 6 amalgamates the structural features of narcipavline and narcikachnine isolated from N. poeticus cv. Pink Parasol ( Šafratová et al., 2018). Moreover, 6 is similar to pallidiflorine, which embodies the galanthamine and tazettine templates ( Codina et al., 1990). Alkaloids 1–5, 7–18, and 20–22 have already been isolated from the genus Narcissus , whereas alkaloids 6 and 19 have been obtained for the first time from a natural source. The scaffold of 19 is already known since it has been previously prepared by reduction of oduline ( Döpke and Bienert, 1966). However, literature and databases do not contain any NMR and MS analysis support for 19. All proton and carbon signals of tetrahydromasonine (19) were completely assigned employing 2D-NMR experiments such as COSY, gHSQC, gHMBC and NOESY, and are summarized in Supplementary Material ( Table S1 View Table 1 ). Key gHMBC and COSY correlations are shown in Fig. 2 View Fig .
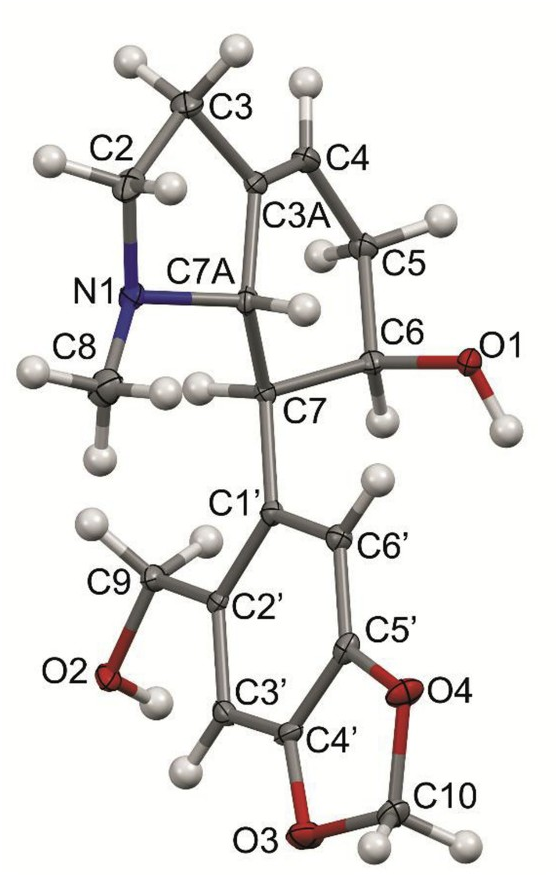
The absolute configuration of tetrahydromasonine has been determined by X-ray study. The solid state structure determined by single crystal X-ray diffraction (sc-XRD) techniques ( Fig. 3 View Fig ) resembles the structures of natural products or their derivatives containing a similar core and arrangement of the skeletal heterocycle ((3a R,5 - S,5a R,11b S,11c R)-5,5a-dihydroxyisopropylidene-1,2,3a,3b,4,5,11b,11c-octahydro-(1,3)dioxolo(4,5-f)pyrrolo(3,2,1-d,e) phenanthridine) ( Haning et al., 2011). In detail, the compound is composed of two bicyclic systems interconnected by a typical single bond ( Fig. 3 View Fig ). The presence of saturated as well as unsaturated bonds is demonstrated by values of interatomic distances recorded in the caption of Fig. 3 View Fig . The presence and orientation of the OH groups lead to the formation of extensive supramolecular architecture promoted by OH ⋯ O and OH ⋯ N bridge connections (Supplementary Material, Fig. S1 View Fig , Table S2 View Table 2 ). According to the IUPAC nomenclature, rings of R 2 2 (16) type promote the formation of 1D chains of molecules .
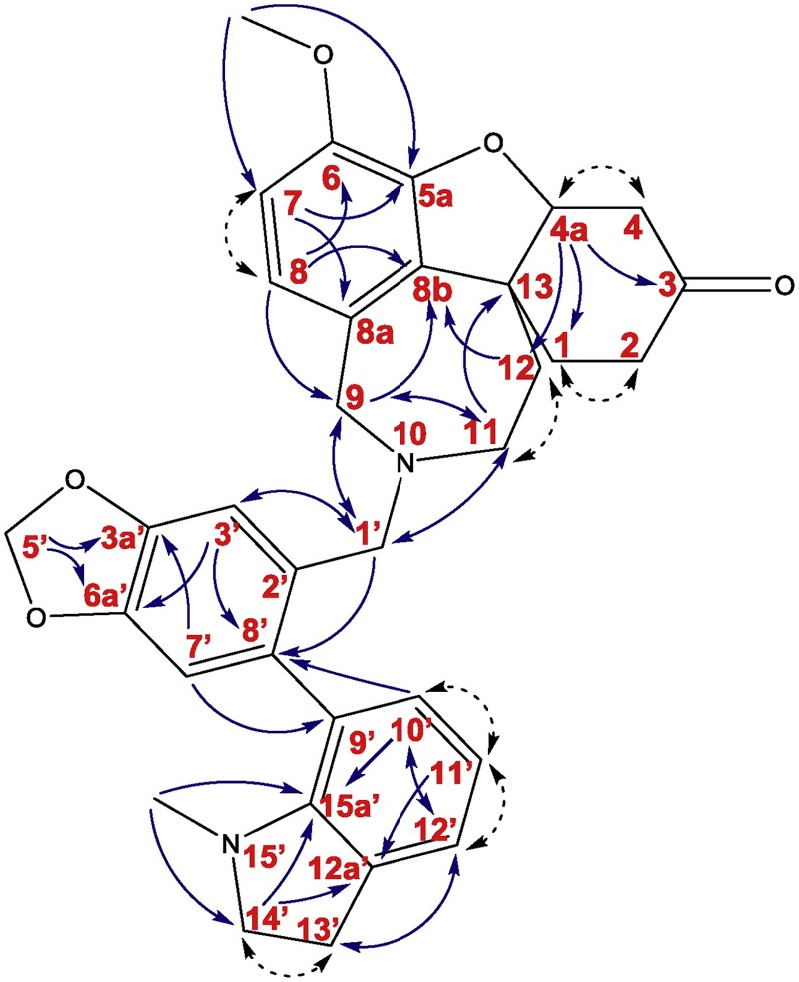
The novel alkaloid 6 was obtained as a light yellow viscous solid. ESI-HRMS of 6 showed a molecular ion peak [M + H] + at m/z 539.2549, corresponding to formula C 33 H 35 N 2 O 5 (calc. 539.2541). 1 H NMR and 13 C NMR spectra showed signals apparently resembling the structural features of 6. The sample was isolated as a mixture of two diastereoisomers. The 1 H NMR spectra allowed to detect the diastereomeric ratio 1:1.1 (isomer A: isomer B). The 1 H NMR data of 6 ( Table 1 View Table 1 ) revealed the presence of 68 protons (34 protons for each isomer) with fourteen of them in the aromatic region and the rest of the signals in the aliphatic area. Four aromatic protons were singlets (δH 7.01, H3′ and 6.74, H7′ for isomer A; 7.07, H3′ and 6.77, H7′ for isomer B), typical for 1, 2, 4, 5-tetrasubstituted benzene ring of each isomer. Four aromatic signals were doublets with J = 8.0 Hz (δH 6.65, H7 and δH 6.44, H8 for isomer A; δH 6.62, H7 and δH 6.56, H8 for isomer B). The other aromatic doublets with J = 7.4 Hz (δH 7.03, H12′ and δH 6.83, H10′ for isomer A; ְ δH 7.03, H12′ and δH 6.71, H10′ for isomer B) and two triplets with J = 7.4 Hz (ְ δH 6.68, H11′ isomer A; δH 6.62, H11′ isomer B) proposed the structural fragments of 1, 2, 3-trisubstituted benzene ring. The signals in the upfield region were mostly multiplets. In the 13 C NMR spectrum of 6, 58 carbon atoms were detected and assigned using gHSQC data. Eight carbons have shown identical positions of resonance for both diastereoisomers, namely: one signal for a carbonyl carbon (δC 209.2, C3), three oxygenated quaternary sp 2 carbons (δC 146.9, C3a’; δC 146.7, C5a; δC 143.6, C6), three quaternary sp 2 carbons (δC 132.0, C8b; δC 129.9, C8a; δC 122.8, C2′) and one sp 2 methine (δC 117.9, C11′). Signals that were separate for each of the isomers include two methoxy groups (δC 55.96, C6-OCH 3 isomer A; δC 55.95, C6-OCH 3 isomer B), two NCH 3 groups (δC 38.8, N2′- CH 3 isomer A; δC 38.7, N2′- CH 3 isomer B), twos -O-CH 2 -O- groups (δC 101.0, C5′ isomer A; δC 100.9, C5′ isomer B), twelve sp 2 methines (δC 130.4, C10′ and δC 123.22, C12′ and δC 122.23, C8 and δC 110.9, C7 and δC 110.5, C7′ and δC 108.7, C3′ for isomer A; δC 130.2, C10′ and δC 123.24, C12′ and δC 122.20, C8 and δC 111.0, C7 and δC 110.3, C7′ and δC 108.4, C3′ for isomer B), two oxygenated methines (δC 88.3, C4a isomer A; δC 88.2, C4a isomer B), eight nitrogenated methylenes (δC 57.6, C9 and δC 57.1, C14′ and δC 54.9, C1′ and δC 52.3, C11 for isomer A; δC 57.9, C9 and δC 56.9, C14′ and δC 53.7, C1′ and δC 51.1, C11 for isomer B) and ten methylene groups (δC 40.05, C4 and δC 37.4, C12 and δC 35.7, C2 and 29.7, C1 and δC 28.6, C13′ for isomer A; δC 40.03, C4 and δC 36.4, C12 and δC 35.6, C2 and δC 29.6, C1 and δC 28.5, C13′ for isomer B). The 13 C-NMR spectrum also exhibited two oxygenated quaternary carbon atoms (δC 146.0, C6a′ isomer A; δC 145.8, C6a′ isomer B), two nitrogenated quaternary sp 2 carbons (δC 150.5, C15a′ isomer A; δC 150.4, C15a′ isomer B), six quaternary sp 2 carbons (δC 133.6, C8′ and δC 131.7, C9′ and δC 131.01, C12a′ for isomer A; δC 133.4, C8′ and δC 131.4, C9′ and δC 131.05, C12a′ for isomer B), and two quaternary sp 3 carbon atoms (δC 47.5, C13 isomer A; δC 47.4, C13 isomer B). Signals have been successfully determined to the individual isomers. The 2D experiments such as gHMBC and gCOSY unequivocally assigned the constitution of compound 6 ( Fig. 4 View Fig ). The results allowed identification of three sub-structural fragments ( Fig. 4 View Fig ): fragments A, B, and C. Fragment A was attached to fragment B via a methylene group (C1′) connecting the nitrogen of fragment A with carbon 2′ of fragment B. Connection of carbon 9′ of N -methylindoline to carbon 8′ of fragment B was determined by key gHMBC correlations.
| R |
Departamento de Geologia, Universidad de Chile |
| S |
Department of Botany, Swedish Museum of Natural History |
| OH |
Agricultural Museum of Praha |
| O |
Botanical Museum - University of Oslo |
| N |
Nanjing University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.