Microlepidogaster arachas Martins, Calegari & Langeani
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3608.5.6 |
|
publication LSID |
lsid:zoobank.org:pub:6A7F1EA0-DDAA-41BE-B9D6-E2F0F0636963 |
|
DOI |
https://doi.org/10.5281/zenodo.5621323 |
|
persistent identifier |
https://treatment.plazi.org/id/5C43E914-FFE1-FFAC-B59C-FB5EFD22FB96 |
|
treatment provided by |
Plazi |
|
scientific name |
Microlepidogaster arachas Martins, Calegari & Langeani |
| status |
sp. nov. |
Microlepidogaster arachas Martins, Calegari & Langeani View in CoL , sp. nov.
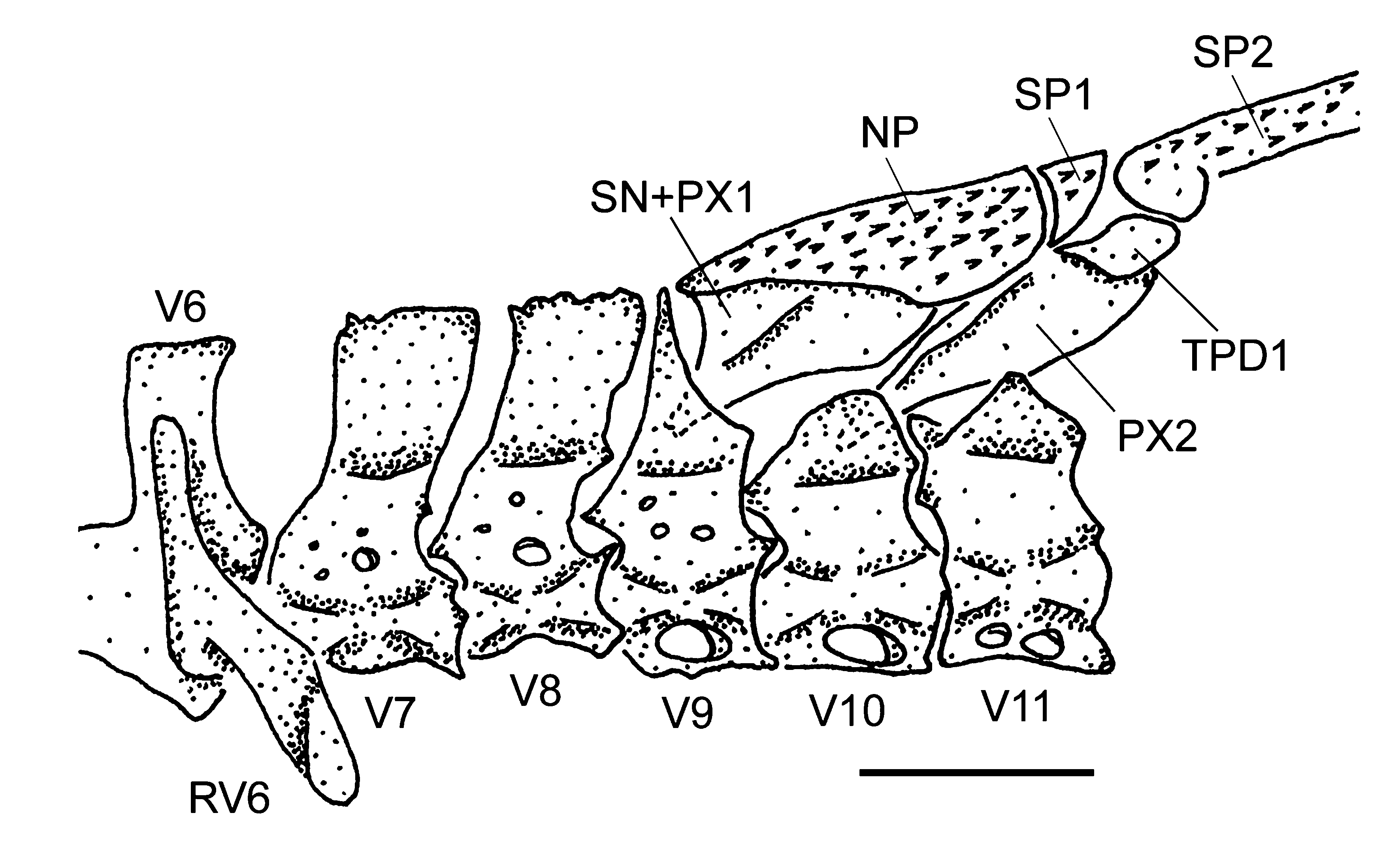
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ; Tables 1–2 View TABLE 1 View TABLE 2 )
Type material. Holotype. DZSJRP 2999, male, 36.7 mm SL, Brazil, Minas Gerais State, stream at Sacramento Municipality, tributary to rio Araguari at road from Sacramento to Araxá, rio Paranaíba drainage, upper rio Paraná basin, 19°49’11”S 47°16’0”W, 13 Aug 1998, F. Langeani & J. I. Montoya-Burgos.
Paratypes. All from Brazil, rio Paranaíba drainage, upper rio Paraná basin. AMNH 256352, 3, 20.6–36.2 mm SL; DZSJRP 15808, 31 (3 c&s), 15.9–38.4 mm SL; LBP 10882, 3, 22.8–35.3 mm SL; MNRJ 39917, 3, 22.5–35.6 mm SL; MZUSP 110976, 3, 22.0– 35.7 mm SL, collected with holotype. DZSJRP 5548, 11 (2 c&s), 15.6–36.3 mm SL, Minas Gerais State, Perdizes Municipality, unnamed stream at dirt road, near BR-262 road, tributary to rio Araguari, 19°36’49”S 47°26’45”W, 20 May 2003, F. Langeani et al. DZSJRP 8683, 6, 26–36.1 mm SL, Minas Gerais State, Monte Carmelo Municipality, córrego Rancharia at Castelhana farm 1, tributary to rio Perdizes, dirt road near BR-262 road, 18°52’14”S 47°23’47”W, 5 Sep 2006, F. Langeani et al. DZSJRP 8743, 9, 23.1–33.4 mm SL, Minas Gerais State, Araxá Municipality, córrego Santo Antônio, affluent to rio Capivara, tributary to rio Araguari, at dirt road, near BR-262 road, from Araxá to Uberaba, 19°34’43”S 47°9’20”W, 7 Sep 2006, F. Langeani et al. DZSJRP 9078, 17 (1 c&s), 35.5–43.4 mm SL, Minas Gerais State, Patrocínio Municipality, stream at country road, tributary to rio Dourados, left on Patrocínio-Coromandel road, 18°54’16”S 46°58’4”W, 11 Aug 2006, F. Langeani & F. R. Carvalho. DZSJRP 9082, 5, 24–33.1 mm SL, Minas Gerais State, Perdizes Municipality, unnamed stream, tributary to rio Araguari at dirt road, right on BR-262 road from Araxá to Uberaba, 19°35’30”S 47°25’22”W, 13 Aug 2006, F. Langeani & F. R. Carvalho. MCP 28333, 5 (1 c&s), 24–37.2 mm SL, Minas Gerais State, Ibiá Municipality, unnamed stream affluent to rio Quebra-Anzol, tributary to rio Araguari, at road from Ibiá to Argenita, 19°38’27”S 46°40’33”W, 27 Jan 2001, C. Lucena et al. MCP 28330, 16 (1 c&s), 27–40.4 mm SL, Minas Gerais State, Rio Paranaíba Municipality, unnamed stream, five kilometers from Rio Paranaíba Municipality, 19°09’05”S 46°15’47”W, 26 Jan 2001, C. Lucena et al. MCP 28319, 19 (3 c&s), 17.4–41 mm SL, Goiás State, Davinópolis Municipality, córrego Grande, at road Davinópolis to Paranaíba ferry, about one kilometer of Davinópolis, 18°09’21”S 47°33’24”W, 22 Jan 2001, C. Lucena et al. MCP 28343, 6, 26–37 mm SL, Minas Gerais State, Argenita Municipality, unnamed stream on Argenita-Pratinha road, at Fazenda Santa Rosa, 19°42’07”S 46°38’56”W, 27 Jan 2001, C. Lucena et al. MCP 28359, 30, 23.8–37.7 mm SL, Minas Gerais State, Cruzeiro Fortaleza Municipality, córrego Jacú, at Rio Serra do Salitre-Brejo Bonito road, rio Quebra-Anzol basin, 19°00’23”S 46°39’37”W, 26 Jan 2001, C. Lucena et al. MCP 28280, 7, 24.2–35.8 mm SL, Minas Gerais State, Rio Paranaíba Municipality, ribeirão da Cachoeira, at Rio Paranaíba-Serra do Salitre road, rio Quebra-Anzol basin, 19°11’32”S 46°24’44”W, 26 Jan 2001, C. Lucena et al. MCP 44879, 11, 13.3–38.2 mm SL, Minas Gerais State, Patrocínio Municipality, unnamed stream, tributary to rio Quebra-Anzol, tributary at UHE Ponte Nova, 19°18’22”S 47°07’15”W, 14 Sep 2009, N. T. Junqueira. MCP 44878, 11, 21.8–35.5 mm SL, Minas Gerais State, Perdizes Municipality, unnamed stream, tributary to rio Quebra-Anzol, tributary at UHE Ponte Nova, 19°14’50”S 47°08’23”W, 15 Sep 2009, N. T. Junqueira. MCP 47026, 28, 25.2–38.1 mm SL, Minas Gerais State, Arax Municipality, córrego Dantas, tributary to rio Araguari, at BR-452 road, between Araxá and Uberaba, 19°31’13”S 47°05’35.3”W, 23 Jan 2012, R. Reis et al.
Diagnosis. Microlepidogaster arachas can be diagnosed from all congeners (except M. perforatus ) by having the anterior portion of compound supraneural plus first dorsal-fin proximal radial contacting the neural spine of ninth vertebra ( Fig. 2 View FIGURE 2 ) (vs. compound supraneural plus first dorsal-fin proximal radial contacting neural spine of 10th or 11th vertebra in M. longicolla , and seventh vertebra in M. dimorpha ). The new species is distinguished from M. perforatus by having 18–29 dentary teeth (vs. 11–15); median series of lateral plates complete, reaching caudalpeduncle end, and continuous lateral line (vs. median series of lateral plates terminating two plates before the end of the caudal peduncle, with non-perforated and missing plates in the middle of the series); and 20–24 mid-dorsal plates (vs. 9–13). Additionally, M. arachas can be distinguished from M. perforatus and M. dimorpha by having anterior portion of rostral plates with small pointed odontodes (vs. small rounded leaf-shaped odontodes); pectoral axillary slit present only in juveniles, absent in adults specimens (vs. pectoral axillary slit persistent, present in both juveniles and adults); and 21–24 mid-ventral plates (vs. 19–20 plates in M. perforatus , and 17–20 plates in M. dimorpha ). Finally, the new species also differs from M. perforatus and M. longicolla by presenting mid-dorsal series of lateral plates surpassing the vertical through dorsal-fin length (vs. mid-dorsal plate series reduced, reaching the vertical through dorsal-fin base, however never surpassing the dorsal-fin length); and first rib attached to seventh vertebra (vs. first rib attached to 10th or 11th vertebra).
Description. Morphometric and meristic data in Tables 1 View TABLE 1 and 2 View TABLE 2 . Dorsal body profile slightly convex from tip of snout to tip of supraoccipital; almost straight to caudal-fin origin. Ventral body profile almost straight from tip of snout to pelvic-fin origin; ascending from pelvic-fin origin to end of anal-fin base; straight to caudal-fin origin. Greatest body depth variable, at supraoccipital tip or at dorsal-fin origin. Greatest body width at opercle opening, gradually tapering towards snout and caudal fin. Caudal peduncle ellipsoid in transverse section, slightly flattened dorsally and ventrally. Head shallow; longitudinal crest and well-developed odontodes absent. Anterior margin of snout rounded in dorsal view; tip of snout with variable coverage, from many small plates to a pair of rostral plates, but frequently with naked area in its most anterior portion, more evident in juveniles; anterior odontodes small and pointed, equal in size to other on remainder head and body. Lateral plate series with enlarged odontodes concentrated along posterior plate margin. Odontodes of head and body not forming conspicuous rows. Eye small, dorsolaterally placed, not visible in ventral view. Iris operculum present. Compound pterotic quadrangular in shape, its posterior extension poor-developed, far from rib of sixth vertebra; pterotic fenestrae most irregular in shape and variable in size, small in dorsal and large in ventral portion of bone. Infraorbital canal entering infraorbital series via sphenotic. Supraoccipital not contributing to dorsal wall of swimbladder capsule.
Body entirely covered by dermal plates, except on ventral part of head, region overlying opening of swimbladder capsule, around pelvic-fin origin and region in front of urogenital opening. Abdomen generally entirely covered by small-sized plates randomly distributed.
Lips roundish, papillose; lower lip larger than upper lip, not reaching pectoral girdle; papillae gradually smaller to lip edge. Maxillary barbel reduced, free from oral disk. Teeth slender and bifid; median cusp larger and rounded, lateral smaller and pointed. Premaxillary teeth 16–30 (20/27). Dentary teeth 18–29 (22). Premaxillary and dentary accessory teeth present only in juveniles, under 23.0 mm SL.
Dorsal-fin rays ii,6–7(7); originating approximately at vertical through middle of pelvic-fin length; tip of adpressed rays surpassing vertical through middle of anal-fin length; spinelet small, somewhat triangular in shape, locking mechanism non-functional. Anterior portion of compound supraneural plus first dorsal-fin proximal radial contacting neural spine of ninth vertebra. Pectoral-fin rays i,5–6(6); originating immediately behind opercular opening; tip of adpressed rays surpassing vertical through end of pelvic-fin base. Cleithrum and coracoid exposed only laterally, median portion covered by small plates. Arrector fossae partially enclosed by ventral lamina of coracoids, opening relatively ample, extending laterally halfway towards pectoral-fin base; opening region of arrector fossae frequently covered by small abdominal plates in adults, these plates eventually fused to each other or to pectoral girdle ( Fig. 3 View FIGURE 3 ). Pectoral axillary slit present only in juveniles. Pelvic-fin rays i,5; unbranched ray shorter than branched rays. Anal-fin rays i,5–6(5). Caudal-fin rays i,12–14(14),i; concave; lobes equal in size; 4 dorsal and 3–5(3) ventral procurrent rays. Adipose fin and azygous plates absent. Median lateral plate series 25–28(27); complete from compound pterotic to caudal-fin base. Vertebrae 31.
Coloration in alcohol. Ground color of dorsal surface of head and body light to median brown, darker along head; pale-yellow, mostly unpigmented ventrally. Dark brown stripe inconspicuous on lateral surface of head and body, beginning laterally on snout tip and extending to end of caudal peduncle. Four dorsal dark brown saddles, mostly restricted along dorsal midline and barely extending laterally and ventrally, sometimes fused to each other forming dark brown mid-dorsal line from dorsal-fin origin to end of caudal peduncle. First saddle at dorsal-fin origin, second behind dorsal-fin base, third and fourth (most inconspicuous) between dorsal and caudal fins. Head with two light stripes from snout tip to compound pterotic, passing through nares, eyes, and dorsal orbital rim.
Ventrolateral margin of head from snout tip to opercle and pectoral-fin insertion creamy white. All fins with hyaline membranes and light brown rays, slightly darker and more conspicuous on unbranched rays. Caudal fin ranging from almost entirely dark brown with distal border hyaline, in most melanic specimens, to median brown, except for distal tip of rays and small round areas on median portions of dorsal and ventral lobes.
Premaxillary teeth 16(1), 18(1), 20(4), 21(2), 22(2), 23(3), 24(3), 25(3), 26(1), 27(4)*, 16–30 20/27
28(2), 29(3), 30(1)
Dentary teeth 18(3), 19(3), 20(3), 21(4), 22(5)*, 23(2), 24(3), 25(1), 26(1), 27(2), 18–29 22
29(3)
Sexual dimorphism. Males with a conspicuous urogenital papillae immediately posterior to anus (vs. absent in females); an expanded flap of skin on dorsal surface of first pelvic-fin ray (vs. absent in females); and pelvic fin almost reaching anal-fin origin (vs. pelvic fin far from anal-fin origin in females).
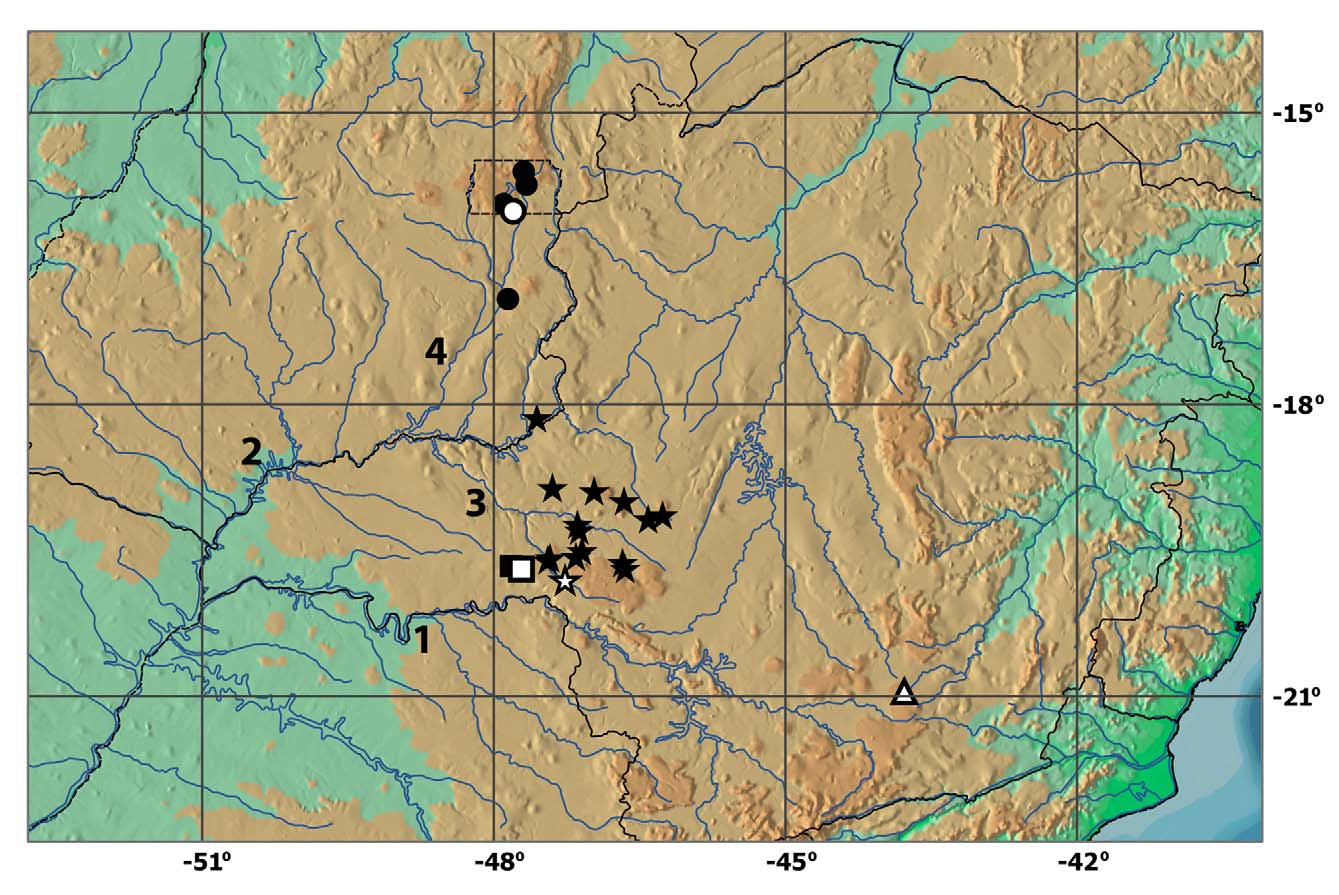
Distribution. Microlepidogaster arachas is known from tributaries to rio Araguari, rio Perdizes, and rio Dourados, all pertaining to the rio Paranaíba drainage, upper rio Paraná basin ( Fig. 4 View FIGURE 4 ).
Remarks. Microlepidogaster arachas is widely distributed at rio Paranaíba basin, its extent of occurrence is about 14.016 km 2 (measured by the minimum convex polygon, using GeoCat software – Geospatial Conservation Assessment Tool). The species is common and abundant (known from 15 localities), and we did not recognize any threats that may endanger it. Therefore, following IUCN criteria, we suggest that this species would fit the Least Concern (LC) category.
Etymology. The specific epithet arachas is a reference to the native people Arachás who once lived in the area drained by the rio Araguari (rio das Velhas), type-locality of the new species, and were exterminated by the Caiapós in 1750s. In the Tupi language Araxá means high place where sun can be seen first, thus Arachás were the ones that inhabited the highlands of southeastern Minas Gerais State. A noun in apposition.
Discussion
Schaefer (1998) suggested five non-exclusive synapomorphies of Microlepidogaster , and among these features M. arachas shares only the dorsal fin positioned posteriorly in the body (synapomorphy modified by Calegari & Reis 2010), presenting the compound supraneural plus first dorsal-fin proximal radial (SN+PX1) contacting the neural spine of ninth vertebra (char. 26: state 1; Schaefer 1998). This synapomorphy can also be observed in M. perforatus and M. longicolla , although the contact of SN+PX 1 may occur in the eighth or ninth vertebra for the former species, and in the 10th or 11th for the latter one. This condition is absent only in M. dimopha , which presents the SN+PX1 contacting mainly the neural spine of seventh vertebra but with the posterior extension contacting also the eighth centrum. The presence of a pair of rostral plates (char. 34: state 1; Schaefer 1998) is another synapomorphy of the genus and is shared only by M. perforatus and M. dimorpha . Microlepidogaster arachas is polymorphic for this character (ranging from many platelets or just a pair of median rostral plates, frequently with a naked area at anterior portion of snout), whereas M. longicolla always presents the anterior portion of snout naked. Among the other three remaining synapomorphies suggested by Schaefer (1998), the presence of a pair of anterior processes in the supraneural (char. 28: state 1; Schaefer 1998), and the median series of lateral plates truncated in the posterior portion, without the last one or two plates, such that the last plates of dorsal and ventral series contact each other in the midline (char. 33: state 1; Schaefer 1998), seem to be autapomorphies of the type-species, M. perforatus , as already suggested by Calegari & Reis (2010) and Martins & Langeani (2011b). Additionally, all species of Microlepidogaster share the presence of the levator crest on the hyomandibula (char. 14, basal state for this character), which Schaefer (1998) considered as absent in M. perforatus .
Microlepidogaster arachas also shares the dorsal fin located more posteriorly relative to the supraoccipital with Rhinolekos Martins & Langeani, 2011 and Epactionotus Reis & Schaefer, 1998 , a feature that seems to have had independent origins within the Hypoptopomatinae . The new species can be easily distinguished from Rhinolekos by the absence of the lateronasal plate (vs. presence of lateronasal plate in all species of the genus. From Epactionotus , M. arachas differs by presenting the tip of snout with many small plates or a pair of rostral plates not ventrally deflected, bearing small odontodes (vs. a single rostral plate ventrally deflected, bearing welldeveloped odontodes); a fleshy flap on dorsal surface of first pelvic-fin ray in males (vs. absent) and by dentary and premaxillary accessory teeth absent in adults (vs. presence of accessory teeth in both, juvenile and adult specimens, see Reis & Schaefer 1998).
Furthermore, M. arachas is morphologically similar to M. longicolla by frequently presenting small plates in the snout, leaving a naked area in its most anterior portion, and pectoral axillary slit and accessory teeth in premaxillary and dentary only in juvenile specimens. According to Martins (2012), these features are observed for the most of Neoplecostominae (such as Neoplecostomus Eigenmann & Eigenmann, 1888 , and Pareiorhina Gosline, 1947 ), Delturinae (such as Hemipsilichthys Eigenmann & Eigenmann, 1889 ), and some basal members of Hypoptopomatinae (such as Rhinolekos and Pseudotocinclus Nichols, 1919 , and Parotocinclus jumbo Britski & Garavello, 2002 , the last just for the pectoral axillary slit) which suggest that these characteristics may be plesiomorphic within the subfamily. Thus, M. longicolla and M. arachas are probably basal members within Microlepidogaster , and the genus is likely to be basal within the subfamily.
The occurrence of M. arachas and M. longicolla in the headwaters of the rio Paranaíba basin, also observed in Rhinolekos , could be another indication of the basal position of Microlepidogaster within Hypoptopomatinae . The Central Brazilian shield was already suggested by many authors to be an ancestral area for many fish groups (Ribeiro et al. 2004; Ribeiro 2006; Menezes et al. 2008; Lima & Ribeiro 2011). Additionally, the occurrence of Plesioptopoma Reis, Pereira & Lehmann, 2012 in the headwaters of the rio São Francisco, and of Gymnotocinclus Carvalho, Lehmann & Reis, 2008 in the headwaters of the rio Tocantins, suggests that the central Brazilian shield shelters the most basal members of Hypoptopomatinae , and likely to be the area where the subfamily originated.
Moreover, Pavanelli & Britski (1999) suggest that the rio Paranaíba basin has high endemism, with an ichthyofauna somewhat distinct from that of the rest of the upper rio Paraná basin. This seems to be the case of some hypoptopomatine species, such as M. arachas , M. longicolla , Hisonotus piracanjuba Martins & Langeani, 2012 , and Rhinolekos species, all recently described and endemic to this portion of the basin.
Comparative material. Microlepidogaster dimorpha , DZSJRP 8750, 19 paratypes (2 c&s), 19.8–37.7 mm SL; DZSJRP 10543, holotype, 37.6 mm SL; DZSJRP 12332, 17 paratypes (2 c&s), 20.3–34.1 mm SL. M. longicolla , DZSJRP 12453, 5 paratypes, 40.8–44.8 mm SL; MCP 44877, holotype, 39.8 mm SL; MCP 23323, 13 paratypes (5 c&s), 18.5–42.5 mm SL; MCP 23322, 10 paratypes, 18.1–36.5 mm SL; MCP 23324, 1 partype, 38.3 mm SL; MCP 23325, 12 paratypes, 19.3–41.2 mm SL; AMNH 251432, 5 paratypes, 23.5–35.7 mm SL; LISDEBE 2662, 3 paratypes, 33.3–36.8 mm SL. M. perforatus , MCZ 8181, holotype, 32 mm SL; MCP 17717, 4 (1 c&s), 14.7–34.5 mm SL; MNRJ 31886, 13 (2 c&s), 27.6–32.9 mm SL; ANSP 174718, 1 (1 c&s), 28–32.4 mm SL.
Acknowledgements
We are grateful to Roberto E. Reis for suggestions that improved the manuscript. The authors were supported by fellowships from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2011/21728-7 to FOM and 2004/00545-8 to FL), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 305.946/2011- 0 to FL and 134901/2008-8 and 140439 /2011-0 to BBC).
References
Britski, H.A. & Garavello, J.C. (2003) Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia, 3, 588 –593. http://dx.doi.org/10.1643/CI-02-23R
Calegari, B.B. & Reis, R.E. (2010) A new species of Microlepidogaster (Siluriformes: Loricariidae: Hypoptopomatinae) from the upper rio Paraná basin, Brazil. Neotropical Ichthyology, 8 (3), 625–630.
Eigenmann, C.H. & Eigenmann, R.S. (1889) Preliminary notes on South American Nematognathi, II. Proceedings of the California Academy of Sciences, 2, 28–56.
Ferreira, K.M. & Ribeiro, A.C. (2007) Corumbataia britskii (Siluriformes: Loricariidae: Hypoptopomatinae) a new species from the upper Rio Paraná basin, Mato Grosso do Sul, Central Brazil. Zootaxa 1386, 59–68.
Lima, F.C.T. & Ribeiro, A.C. (2011) Continental-Scale Tectonic Controls of Biogeography and Ecology. In: Albert, J. S. & Reis, R. E. (Eds), Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley, pp. 145–164. http://dx.doi.org/10.1525/california/9780520268685.003.0009
Martins, F. O. (2012) Análise Filogenética e Revisão Taxonômica de Pseudotothyris Britski & Garavello, 1984 (Loricariidae: Hypoptopomatinae). M. Sc. Thesis. Universidade Estadual Paulista Júlio de Mesquita Filho, São José do Rio Preto, 188 pp.
Martins, F. O. & Langeani, F. (2011 a) Rhinolekos, a new genus with three new species of Hypoptopomatinae (Siluriformes: Loricariidae) from upper rio Paraná. Neotropical Ichthyology, 9 (1), 65–78. http://dx.doi.org/10.1590/S1679-62252011000100004
Martins, F. O. & Langeani, F. (2011 b) Microlepidogaster dimorpha, a new species of Hypoptopomatinae (Siluriformes: Loricariidae) from the upper rio Paraná system. Neotropical Ichthyology, 9 (1), 79–86. http://dx.doi.org/10.1590/S1679-62252011000100005
Martins, F. O. & Langeani, F. (2012) Hisonotus piracanjuba, a new species of Hypoptopomatinae (Siluriformes: Loricariidae) from the rio Paranaíba, upper rio Paraná system, central Brazil. Ichthyological Exploration of Freshwaters, 23 (1), 29–36.
Menezes, N.A., Ribeiro, A.C., Weitzman, S. & Torres, R.A. (2008) Biogeography of Glandulocaudinae (Teleostei: Characiformes: Characidae) revisited: phylogenetic patterns, historical geology and genetic connectivity. Zootaxa, 1726, 33–48.
Pavanelli, C.S. & Britski, H.A. (1999) Description of a new species of Steindachnerina (Teleostei: Characiformes: Curimatidae) from the upper Rio Paraná basin, Brazil. Ichthyological Exploration of Freshwaters, 10 (3), 211–216.
Reis, R.E. & Schaefer, S.A. (1998) New Cascudinhos from Southern Brazil: Systematics, Endemism, and Relationships (Siluriformes, Loricariidae, Hypoptopomatinae). American Museum Novitates, 3254, 1–25.
Ribeiro, A.C. (2006) Tectonic history and the biogeography of the freshwater fishes from the coastal drainages of eastern Brazil: an example of faunal evolution associated with a divergent continental margin. Neotropical Ichthyology, 4 (2), 225–246. http://dx.doi.org/10.1590/S1679-62252006000200009
Ribeiro, A.C., Benine, R.C. & Figueiredo, C.A. (2004) A new species of Creagrutus Günther (Teleostei: Ostariophysi: Characiformes), from the upper Rio Paraná basin, central Brazil. Journal of Fish Biology, 64, 597 –611. http://dx.doi.org/10.1111/j.1095-8649.2004.00324.x
Ribeiro, A.C., Carvalho M. & Melo, A.L.A. (2005) Description and relationship of Otothyropsis marapoama, a new genus and species of Hypoptopomatinae catfish (Siluriformes: Loricariidae) from rio Tietê basin, southeastern Brazil. Neotropical Ichthyology, 3 (4), 489–498. http://dx.doi.org/10.1590/S1679-62252005000400006
Schaefer, S.A. (1997) The Neotropical cascudinhos: Systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proceedings of the Academy of Natural Sciences of Philadelphia, 148, 1–120.
Schaefer, S.A. (1998) Conflict and Resolution: Impact of New Taxa on Phylogenetic Studies of the Neotropical Cascudinhos (Siluroidei: Loricariidae). In: Malabarba, L. R., Reis, R. E., Vari, R. P., Lucena, Z. M. S. & Lucena, C. A. S. (Eds), Phylogeny and Classification of Neotropical Fishes. Edipucrs, Porto Alegre, pp. 375–400.
Taylor, W.R. & Van Dike, G.C. (1985) Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9 (2), 107–119.
TABLE 1. Morphometric data for Microlepidogaster arachas, holotype (H) and 29 paratypes; range includes holotype. SD = standard deviation. Diagnostic values for M. longicolla are in italic, and bold for M. perforatus.
| Character | H | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Standard length (mm) | 36.7 | 29.6 | 43.35 | ||
| Percents of standard length | |||||
| Predorsal length | 47.7 | 43.7 | 50.4 | 47.0 | 1.6 |
| Preanal length | 57.3 | 55.7 | 61.6 | 58.2 | 1.4 |
| Prepectoral length | 25.2 | 22.6 | 27.0 | 24.9 | 1.1 |
| Prepelvic length | 38.2 | 33.9 | 40.3 | 37.4 | 1.7 |
| Thoracic length | 16.8 | 13.4 | 18.7 | 15.8 | 1.4 |
| Abdominal length | 19.8 | 18.4 | 24.5 | 21.5 | 1.3 |
| Caudal-peduncle depth | 9.5 | 8.1 | 10.3 | 9.4 | 0.6 |
| Caudal-peduncle length | 35.8 | 32.3 | 36.4 | 35.0 | 1.2 |
| Caudal-peduncle width | 10.0 | 7.7 | 10.3 | 9.1 | 0.7 |
| Head length | 31.6 | 29.1 | 33.4 | 31.1 | 1.2 |
| Head depth | 15.3 | 14.0 | 16.3 | 15.0 | 0.7 |
| Cleithral width | 23.6 | 21.1 | 27.2 | 23.3 | 1.4 |
| Base of dorsal fin length | 11.5 | 9.2 | 13.1 | 11.4 | 0.9 |
| Dorsal-fin unbranched ray length | 20.3 | 18.7 | 23.3 | 20.2 | 1.0 |
| Pectoral-fin unbranched ray length | 19.7 | 18.1 | 23.4 | 20.5 | 1.4 |
| Pelvic-fin unbranched ray length | 16.1 | 13.9 | 20.1 | 16.5 | 1.7 |
| Anal-fin unbranched ray length | 17.1 | 14.2 | 20.3 | 17.4 | 1.2 |
| Dorsal to anal fin length | 19.3 | 18.7 | 22.1 | 20.3 | 0.9 |
| Snout-opercle length | 25.1 | 23.1 | 26.4 | 24.8 | 0.9 |
| Percents of head length | |||||
| Head width | 71.2 | 71.2 | 79.5 | 75.6 | 2.4 |
| Head depth | 48.4 | 42.4 | 52.1 | 48.3 | 1.9 |
| Snout length | 53.8 | 51.8 | 57.1 | 54.5 | 1.2 |
| Orbital diameter | 12.8 | 12.2 | 14.9 | 13.5 | 0.9 |
| Interorbital length | 36.8 | 36.3 | 43.2 | 39.8 | 2.0 |
| Maxillary barbel length | 4.5 | 3.0 | 6.0 | 4.7 | 0.8 |
| Prenasal length | 32.4 | 29.5 | 36.8 | 33.7 | 1.5 |
| Internasal length | 8.7 | 7.5 | 11.7 | 9.1 | 1.1 |
| Nostril width | 8.7 | 7.9 | 11.0 | 9.2 | 0.9 |
| Nostril length | 13.8 | 11.3 | 17.1 | 14.1 | 1.6 |
| Suborbital depth | 27.2 | 24.4 | 29.6 | 26.6 | 1.1 |
TABLE 2. Frequency distribution of meristic data for Microlepidogaster arachas, holotype, 29 paratypes in alcohol, and 4 c & s paratypes. Procurrent rays and vertebrae counts were made only in c & s specimens. Holotype values are marked with an asterisk. Diagnostic values for M. perforatus are in bold.
| Character | Frequency distribution | Range Mode |
|---|---|---|
| Dorsal plates | 25(4), 26(11), 27(15)* | 25–27 27 |
| Mid-dorsal plates | 20(9), 21(13), 22(7)*, 24(1) | 20–24 21 |
| Median plates | 25(1), 26(5), 27(16)*, 28(8) | 25–28 27 |
| Mid-ventral plates | 21(6), 22(12), 23(8), 24(4)* | 21–24 22 |
| Ventral plates | 20(1), 21(3), 22(9), 23(11), 24(3)*, 25(1), 26(2) | 20–26 23 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.