Chaoborus albipes ( Johannsen, 1903 ), 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4927.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:942E128B-0A2C-4799-9A8A-B87A1A4FF627 |
|
DOI |
https://doi.org/10.5281/zenodo.4564951 |
|
persistent identifier |
https://treatment.plazi.org/id/5B678789-FFD6-1450-A09D-09D9FB9BFB08 |
|
treatment provided by |
Plazi |
|
scientific name |
Chaoborus albipes ( Johannsen, 1903 ) |
| status |
stat. nov. |
Chaoborus albipes ( Johannsen, 1903) View in CoL , stat. rev.
Corethra albipes Johannsen 1903: 398 View in CoL (original description of female holotype), wing plate 39 fig. 11. Richardson 1912: 202 (redescription of larva, especially in comparison to C. plumicornis var. americana View in CoL (= Chaoborus americanus View in CoL ), description of male, short comments on pupa and adult female, biology), larval antennae fig. 1, male wing fig. 2.1, female wing fig. 2.2
Sayomyia albipes: Felt 1904: 363 (in part, description of female copied from Johannsen 1903, description of pupa and male). Felt 1905: 497 (verbal description of male hypopygium). Dyar 1905: 16 (key to larvae, confused with C. punctipennis View in CoL ).
Chaoborus (Chaoborus) albipes: Dyar & Shannon 1924: 211 View in CoL (comparison to Chaoborus crystallina View in CoL and C. flavicans View in CoL , distribution in USA). Hennig 1968: 74 (list of Nearctic species, literature of larvae and pupae).
Chaoborus albipes: Johannsen 1934: 44 View in CoL (identification key, diagnoses of larva and pupa, var.a. consists of larvae with 25 or more anal fan setae and somewhat broader labral blades), larva habitus plate 43 fig. 166, pupa habitus fig. 167, labral blades fig. 171. Dickinson 1944: 357 (verbal description of adult and larva, perhaps based on earlier references, occurrence in Wisconsin), labral blade fig. 229. Belkin et al. 1966: 22 (location of holotype, type locality, bionomics, as a syn. of C. flavicans View in CoL ).
Sayomyia rotundifolia Felt 1904: 366 View in CoL syn. nov. (description of female, male, larva and pupa), female wing plate 13 fig. 2, male wing plate 13 fig. 3, male hypopygium plate 40 fig. 2. Felt 1905: 497 (verbal description of male hypopygium). Dyar 1905: 16 (key to larvae).
Chaoborus rotundifolia: Johannsen 1934: 43 View in CoL (identification key, following the concept by Felt).
Chaoborus (Chaoborus) rotundifolia: Hennig 1968: 74 View in CoL (list of Nearctic species, literature of larvae and pupae).
Chaoborus crystallina: Matheson 1925: 159 View in CoL ( Sayomyia rotundifolia View in CoL as a new synonym of C. crystallina View in CoL ).
Chaoborus crystallinus: Yamada 1932: 230 View in CoL (redescription of female), habitus photo of adult female on page 230. Matheson 1944: 95 (identification key), male hypopygium plate 10 fig. 4.
Chaoborus (Chaoborus) flavicans: Cook 1956: 23 View in CoL , in part. (redescription of C. (C.) flavicans View in CoL includes both C. flavicans View in CoL and C. albipes View in CoL ), parameres (fig. 16H).
Chaoborus flavicans: Strickman 1980 View in CoL : (in vitro copulation and oviposition), egg mass. fig. 1. Likely the species involved here is C. albipes View in CoL , because material studied was from a “shallow, woodland pond”. Ogawa 2007: (phylogeny), mandible fig. 2.15b, parameres fig. 2.51b. Luoto & Nevalainen 2009: in part. (paleolimnology), mandibles in fig. 2a represent C. albipes View in CoL .
Chaoborus cf. flavicans: Dupuis et al. 2008 View in CoL : (molecular analysis, phylogeny, distribution, ecology, morphology), mandible fig. 2A. An et al. 2010: (molecular phylogeny, partly based on same material as in Dupuis et a. 2008). Taylor et al. 2015: (distribution, ecology). Ballinger et al. 2014: (RNA virus infection, distribution). Ballinger et al. 2017: (RNA virus infection, distribution).
Material examined. Type material. Holotype. [“O.A.J. det 1759” “ Corethra albipes Jo. Ithaca N.Y. 1901 HOLOTYPE no 2968 (partly hand written)” “Cornell University Dept. of Entomology”] white label on slide. [“HOLOTYPE Cornell U. No. 2968”] red label on slide ( CUI). An additional white label is glued on left upper corner, [ Chaoborus flavicans (Meig.) det. E.F. Cook 1951]. The holotype of C. albipes consists of a slide mounted wing only, the rest of the specimen is lost (J.J. Dombroskie, pers.comm.). The holotype was studied from photos.
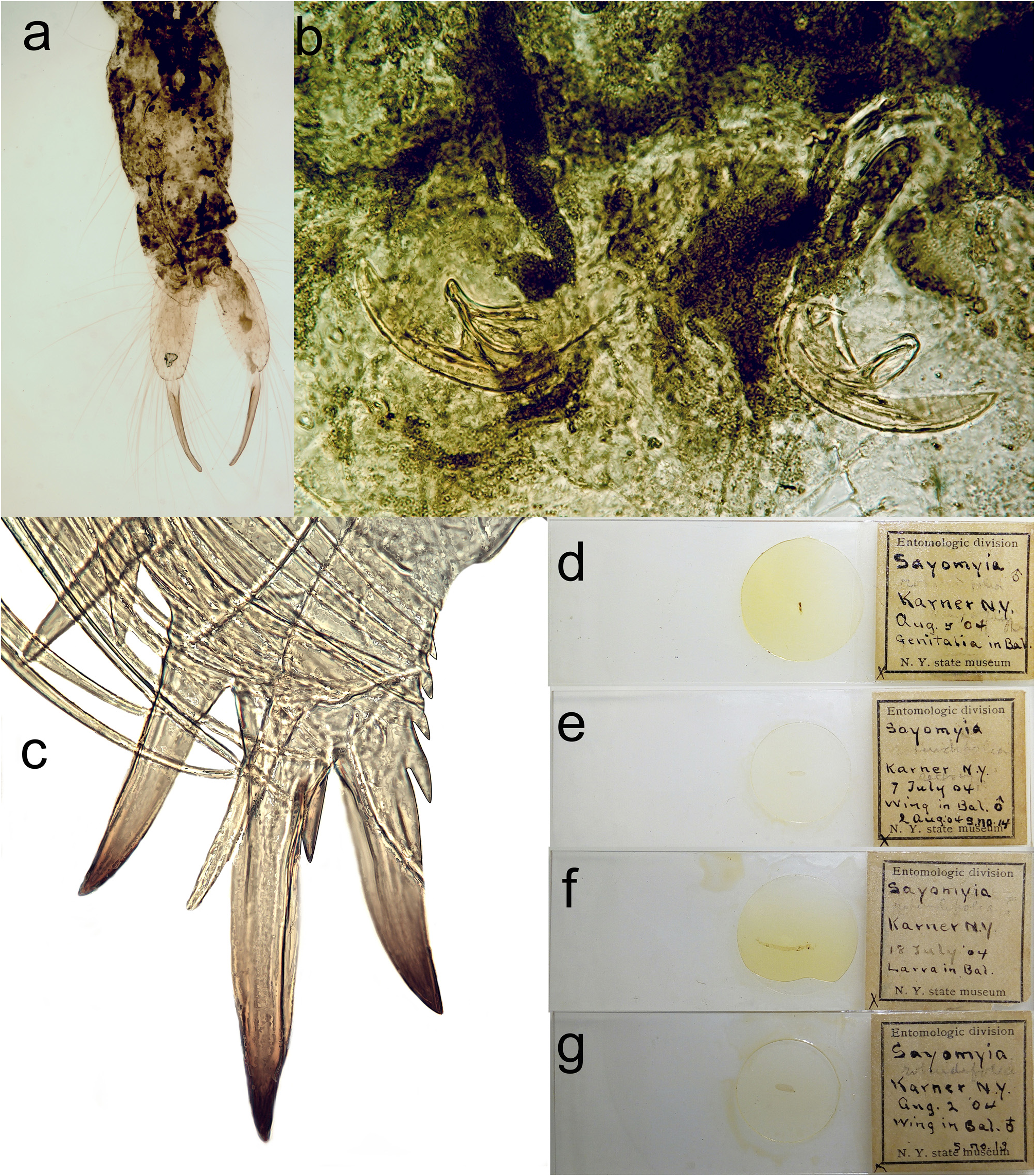
Lectotype of Sayomyia rotundifolia , by present designation (J. Salmela, Fig. 2a,b,d View FIGURE 2 ). [“Entomologic division (printed)” “ Sayomyia rotundifolia ³” “Karner N.Y.” “Aug. 5 ‘04” “Genitalia in Bal. (hand written)” “N.Y. state museum (printed)”]; lectotype of S. rotundifolia consists of a slide mounted male abdominal terminalia. Paralectotypes, by present designation. 1 male, [“Entomologic division (printed)” “ Sayomyia rotundifolia ” “Karner N.Y.” “1 July 04” “Wing in Bal. ³ s.no. 14 (hand written)” “N.Y. state museum (printed)”]; a wing on a slide ( Fig. 2e View FIGURE 2 ). 1 larva, [“Entomologic division (printed)” “ Sayomyia rotundifolia ” “Karner N.Y.” “18 July ‘04” “Larva in Bal. (hand written)” “N.Y. state museum (printed)” ( Fig. 2c,f View FIGURE 2 ); one slide mounted larva, intact, i.e. mouthparts etc. not detached; most likely belong to C. flavicans , not C. albipes . 1 female, [“Entomologic division (printed)” “ Sayomyia rotundifolia ” “Karner N.Y.” “Aug. 2 04” “Wing in Bal. ♀ (hand written)” “S.no.14 (handwritten)” “N.Y. state museum (printed)”]; one slide-mounted female wing ( Fig. 2g View FIGURE 2 ). According to the original description Felt studied only one male specimen, but different dates on the labels indicate that lectotype male and paralectotype male are different specimens.
Other material. Finland. Ab: Salo, Härjänsilmä, Salmela J. leg. 2. V.2020, 6 larvae, NVO.LMM-el-20-5; 1 male e.l., NVO.LMM-el-20-15; 1 male e.l., NVO.LMM-el-20-16; 1 male e.l., NVO.LMM-el-20-17; 1 male e.l., NVO.LMM-el-20-18; 1 male e.l., NVO.LMM-el-20-23; 1 female e.l., NVO.LMM-el-20-27; 1 male e.l., NVO. LMM-el-20-28; 1 male e.l., NVO.LMM-el-20-29; 1 male pupa, e.l., NVO.LMM-el-20-34; 4 males e.p., NVO. LMM-el-20-49; 2 males e.p., NVO.LMM-el-20-55 ( LMM); 1 male e.p. ( ABC). N: Sipoo, Jöusjärv, Salmela J. leg. 2. V.2020, 1 pupa e.l., NVO.LMM-el-20-2; 1 male e.l., NVO.LMM-el-20-19; 1 male e.l., NVO.LMM-el-20-20; 1 male e.l., NVO.LMM-el-20-21; 1 male e.p., NVO.LMM-el-20-31; 1 male e.p., NVO.LMM-el-20-47; 1 female e.p., NVO.LMM-el-20-54 ( LMM). Sipoo, Fallträsk, Salmela J. leg. 2. V.2020, 1 larval and pupal exuviae on slide, adult escaped ( LMM). Ta: Tammela, Kärjensuo 1, Härmä O. leg. 9.IV.2020, 1 male e.l., LG.6092; 1 male e.l., LG.6092; 1 male e.l., LG.6093; 1 male e.l., LG.6094; 1 male e.l., LG.6109; 1 pupa e.l., LG.6110; 1 female e.l., LG.6111; 1 male e.l., LG.6128; 1 male e.l., LG.6129; 1 male e.l., LG.6130; 1 male e.l., LG.6132; 1 male e.l., LG.6164; 1 female e.l., LG.6165; 6 larva, LG.6165 ( FLHM). Tammela, Kärjensuo 2, Härmä O. leg. 9.IV.2020, 1 larva ( FLHM). Tammela, Ammeenpohja, Härmä O. leg. 16.IV.2020, 2 larvae, LG.6113; 1 male e.l., LG.6227; 1 male e.l., LG.6228; 1 female e.l., LG.6229; 1 pupa e.l., LG.6230; 1 male e.l., LG.6231; 1 female e.l., LG.6232; 1 pupa, LG.6234; 1 male e.l., LG.6246 ( FLHM). Tammela, Lehmälammi, Härmä O. leg. 21.IV.2020, 9 larvae, LG.6193; 1 male e.l., LG.6236; 1 male e.l., LG.6237; 1 female e.l., LG.6238; 1 female e.l., LG.6239; 1 male e.l., LG.6240; 1 female e.l., LG.6241; 1 male e.l., LG.6242; 1 female e.l., LG.6252; 1 pupa e.l., LG.6259; 1 pupa e.l., LG.6260 ( FLHM). Tammela, Paskolammi, Härmä O. leg. 30.IV.2020, 5 larvae, LG.6262 ( FLHM). Tammela, Vehkala, Härmä O. leg. 21.IV.2020, 10 larvae, LG.6198; 1 female e.l., LG.6243; 1 female e.l., LG.6244; 1 female e.l., LG.6245; 1 female e.l., LG.6253; 1 male e.l., LG.6255; 1 female e.l., LG.6256; 1 female e.l., LG.6257; 1 pupa e.l., LG.6258 ( FLHM). Jokioinen, Kaitalammi, Härmä O. leg. 13.IV.2020, 10 larva, LG.6126; 5 larvae, LG.6147; 1 male e.l., LG.6169; 1 male e.l., LG.6170; 1 male e.l., LG.6172; 1 male e.l., LG.6173; 1 female e.l., LG.6173; 1 female e.l., LG.6175; 1 male e.l., LG.6176; 1 male e.l., LG.6177; 1 male e.l., LG.6178; 1 male e.l., LG.6179; 1 male e.l., LG.6180; 1 female e.l., LG.6181; 1 pupa e.l., LG.6182; 9 males e.l., LG.6183; 8 females e.l., LG.6184; 1 male e.l., LG.6202; 1 female e.l., LG.6203; 1 female e.l., LG.6204; 1 male e.l., LG.6205; 1 pupa e.l., LG.6226; 1 pupa e.l., LG.6248; 1 pupa e.l., LG.6249; 1 pupa e.l., LG.6250 ( FLHM). Somero, Hossinoja, Härmä O. leg. 10.IV.2020, 1 male e.l., LG.6102; 1 male e.l., LG.6103; 1 male e.l., LG.6104; 1 male e.l., LG.6105; 1 female e.l., LG.6106; 1 female e.l., LG.6107; 1 male e.l., LG.6108; 1 male e.l., LG.6122; 1 male e.l., LG.6124; 4 larva, LG.6125; 1 male e.l., LG.6131; 1 female e.l., LG.6133; 1 pupa e.l., LG.6134; 1 pupa e.l., LG.6166 ( FLHM). Somero, Jorri, Härmä O. leg. 18.IV.2020, 2 larvae, LG.6163 ( FLHM). Somero, Salakkajärvi W, Salmela J. leg. 2. V.2020, 4 larvae, NVO.LMM-el-20-9; 1 male e.l., NVO.LMM-el-20- 12; 1 male e.l., NVO.LMM-el-20-13; 1 male e.l., NVO.LMM-el-20-14; 1 pupa e.l., NVO.LMM-el-20-25; 1 male e.l., NVO.LMM-el-20-26; 5 male e.p., NVO.LMM-el-20-32; 2 male e.p., NVO.LMM-el-20-50; 1 male e.p., NVO. LMM-el-20-56 ( LMM). Somero, Salakkajärvi W, Härmä O. leg. 10.IV.2020, 1 male e.l., LG.6095; 1 larva, LG.6114 ( FLHM). Somero, Äijämö, Härmä O. leg. 18.IV.2020, 1 larva, LG.6156; 1 larva, LG.6158; 1 male e.l., LG.6212; 1 female e.l., LG.6213; 1 male e.l., LG.6214; 1 male e.l., LG.6215; 1 male e.l., LG.6217; 1 pupa e.l., LG.6218; 1 pupa e.l., LG.6219; 1 pupa e.l., LG.6220; 1 pupa e.l., LG.6221; 1 pupa e.l., LG.6222; 1 pupa e.l., LG.6223 ( FLHM). Kouvola, Konttisuo, Salmela J. leg. 6. V.2019, 1 larva, head, tail, respiratory organs of prepupa (taken beneath larval skin) on slide, BOLD, NVO.CUL-2019-26 ( LMM); Kymijoen vesi ja ympäristö leg. 18.XI.2015, 1 larva, head & tail on slide, BOLD, NVO.ins2018-783 ( LMM); 1 larva, head + tail on slide, NVO.CUL-2019-84 ( LMM). Sa: Savonlinna, Ukonlampi, Salmela J. leg. 07. V.2019, 1 male e.l., pinned, larval and pupal exuviae on slide, hypopygium on slide, BOLD, NVO.LMM-el-15; 1 male e.l., pinned, NVO.LMM-el-17; 1 female e.l., larval and pupal exuviae on slide, NVO.LMM-el-42; 1 male e.l., pinned, NVO.LMM-el-57; 1 male e.l., pinned, pupal exuviae on slide, BOLD, NVO.LMM-el-61; 3 males e.p., NVO.LMM-el-64; 1 male e.l., pinned, pupal exuviae and hypopygium on slide, BOLD, NVO.LMM-el-76; 1 male e.l., pinned, NVO.LMM-el-99 ( LMM). Sb: Joroinen, Ulminmäki, Salmela J. leg. 07. V.2019, 1 male e.l., pinned, larval and pupal exuviae on slide, hypopygium on slide, BOLD, NVO.LMMel-10; 1 male e.l., pinned, larval and pupal exuviae on slide, hypopygium on slide, BOLD, NVO.LMM-el-11; 1 male e.l., pinned, larval and pupal exuviae on slide, hypopygium on slide, NVO.LMM-el-13; 1 male e.l., larval and pupal exuviae on slide, hypopygium on slide, NVO.LMM-el-29; 1 female e.p., pinned, pupal exuviae on slide, NVO. LMM-el-39; 1 male e.p., NVO. LMM-el-40; 1 male e.l., pinned, pupal exuviae on slide, NVO.LMM-el-41; 3 males e.p., parameres of two specimens glued on slide, NVO.LMM-el-49; 1 male e.p., pupal exuviae, wings, legs, head, hypopygium on slide, NVO.LMM-el-50 ( LMM). Tb: Toivakka, Kataislammit, Salmela J. leg. 1. V.2020, 1 larva, NVO.LMM-el-20-1; 1 male e.l., NVO.LMM-el-20-42; 1 male e.l., NVO.LMM-el-20-43; 1 pupa e.l., NVO.LMMel-20-44; 1 male e.l., NVO.LMM-el-20-45 ( LMM). Obb: Rovaniemi, Pietarinlampi, Salmela J. leg. 5. VI.2020, 1 male e.p., pupal exuviae and hypopygium on slide, NVO.LMM-el-20-105; 1 male e.p., adult pinned, pupa exuviae on slide, NVO.LMM-el-20-107; 1 male e.p., NVO.LMM-el-20-108 ( LMM). Lkoc: Kittilä, Muotrikkilehto, Salmela J. leg. 27.VII.2019, 1 larva, NVO.LMM-el-608 ( LMM); 1 larva, NVO.LMM-el-610 ( LMM); 1 larva, head & tail on slide, BOLD, NVO.CUL-2019-78 ( LMM); 1 larva, head & tail on slide, BOLD, NVO.CUL-2019-79 ( LMM). Kittilä, Papinmutka, Salmela J. leg. 28.VII.2019, 1 female, pupal exuviae and female in EtOH, BOLD, NVO.LMMel-526 ( LMM). Lkor: Savukoski, Rouvoivanselkä, Salmela J. leg 05. VI.2019, 1 male e.l., larval and pupal exuviae, hypopygium on slide, NVO.LMM-el-206; 1 male e.l., NVO.LMM-el-211; 1 male e.l., larval and pupal exuviae on slide, legs, wings, head on slide, torso EtOH, NVO.LMM-el-212; 1 male e.l., larval and pupal exuviae on slide, legs, wings, head on slide, torso EtOH, NVO.LMM-el-213; 1 male e.l., larval and pupal exuviae on slide, legs, wings, head, hypoopgium on slide, torso EtOH, NVO.LMM-el-214; 1 male e.l., NVO.LMM-el-215; 1 female e.l. pinned, larval & pupal exuviae on slide, NVO.LMM-el-216; 1 male e.l., NVO.LMM-el-217; 1 male e.l., NVO.LMM-el- 218; 1 male e.l., NVO.LMM-el-219; 1 male e.l., NVO.LMM-el-220; 1 female e.l., NVO.LMM-el-221; 1 male e.l., NVO.LMM-el-222; 1 male e.l., LMM-el-223; 1 male e.l., NVO.LMM-el-224; 1 male e.l., NVO.LMM-el-225; 1 male e.l., NVO.LMM-el-226; 1 male e.l., NVO.LMM-el-227; 1 pupa e.l., NVO.LMM-el-228; 1 pupa e.l., NVO. LMM-el-229; 1 male e.l., NVO.LMM-el-230; 1 male e.l., NVO.LMM-el-231; 1 female e.l., NVO.LMM-el-232; 1 female e.l., larval and pupal exuviae on slide, NVO.LMM-el-233; 1 pupa e.l., NVO.LMM-el-234; 1 male e.l., NVO. LMM-el-290; Laine E. & Salmela J. leg. 13. VI.2018, 5 larvae 5 pupae, one teneral hypopogium on slide, NVO. ins2018-420; 10 larvae 3 pupae, one teneral hypopogium on slide NVO.ins2018-421; 1 pupa teneral hypopogium on slide, NVO.ins2018-427; 1 pupa, BOLD, NVO.ins2018-624; 1 pupa, BOLD, NVO.ins2018-625; 1 larva, BOLD, NVO.ins2018-626; 1 larva, BOLD; NVO.ins2018-627; 1 larva, BOLD, NVO.ins2018-628; 1 larva, NVO.ins2018- 681; Laine E. leg. 12.VII.2018, 7 larvae I and II instar, NVO.ins2019-195 ( LMM).
Norway. Buskerud, Kongsberg , Karlshaug - N Svensketjern , 59.5302 9.5642, 495 masl, K.M. Olsen leg. 31. V GoogleMaps .- 26. VI GoogleMaps .2013, 20 males, 1 on slide, J.nr. BAB 470603 ( KMO) .
Russia. South Urals , Osipovka, Salokannel, J. leg. 05.VIII.2018, 1 female, BOLD, NVO.ins2018-457 ( LMM). Primorskiy Kray, Kiparisovo (Tazyozhnyi), 43.4858 131.975 GoogleMaps , E. Zakharov leg. 7.VII.2014, 1 female, BOLD, BIOUG 15361- C01 ( CBG)
Japan. Tokyo, Yotsuya, 35.68 139.72, leg? 1948, 3 males 1 female in EtOH ( NMNS). Sado Island , 38.03 138.33 , I. Tosayabashi leg. 16. VI .1917 , 1 male, pinned hypopygium in microvial ( NMNS). Shiba , Tokio, 35.65 139.74, S. Yamada leg. 20.VIII.1919, 1 male, pinned ( NMNS) . Hokkaido, K. Tanaka leg., no date, 3 males, pinned (two hypopygia in microvial), A-0213, ( NMNS). Matsuyama-shi, Higashino , 33.8411 132.8111 GoogleMaps , J. Oku leg. 19. V .2016 , 1 male, pinned, hypopygium in microvial ( ELEU). Honsu , Niigata, Matsunoyama-Kannonji, Kato D. leg. 13.VIII.2020, 1 male, NVO . JAP-06; 1 male, NVO . JAP-07; 1 male, NVO . JAP-09; 1 male, NVO .JAP-11 ( LMM).
Canada. Ontario, Roseneath L., 44.19 -78.05, H.G. James leg. 1963, 1 male on slide, 38 ( CNC). GoogleMaps Ontario, Carden Alvar, Cameron Ranch, 44.637 -79.058, J. Cossey leg. 6.10.2011, 1 female, BOLD, BIOUG01756 View Materials - G05 ( CBG). GoogleMaps Ontario, Rouge National Urban Park , Toronto Zoo , 43.8223 -79.1897, 125 masl, K. Kerr & A. Sritharan leg. 1.VII.2014, 1 female, BOLD, BIOUG20540 View Materials - A09 GoogleMaps ; 1 male, BOLD, BIOUG20540 View Materials - B02 ( CBG). Ontario, Cambridge, Indian Woods , 43.3736 -80.3652, 304 masl, BIO Collections Staff leg. 14. V GoogleMaps .2015, 1 female, BOLD, BIOUG 22361- C03 ( CBG). Ontario, Charleston Lake Provincial Park, Hemlock Ridge Trail , 44.5054 -76.0275, 108 masl, BIObus leg. 22. VI GoogleMaps .2015, 1 III instar larva, BOLD, BIOUG23326 View Materials - C12 ( CBG). Ontario, Owen Sound, Bayview Escarpment Provincial Park , 44.6337 -80.6983, 319 masl, CBG Collections Staff leg. 10.VII.2014, 1 male, BOLD, BIOUG33751 View Materials - B08 GoogleMaps ; 1 male, BOLD, BIOUG33752 View Materials - A07 ( CBG). GoogleMaps Ontario, Tiny, Awenda Provincial Park, 44.8253 -79.9846, 231 masl, CBG Collections Staff leg. 8.VIII.2014, 1 female, BOLD, BIOUG34258 View Materials - F03 ( CBG). GoogleMaps Ontario, Morpeth , Rondeau Provincial Park , 42.3021 -81.8531, 239 masl, CBG Collections Staff leg. 13. V GoogleMaps .2014, 1 male, BOLD, BIOUG34670 View Materials - D06 ; 1 male, BOLD, BIOUG34670 View Materials - G06 ; 1 female, BOLD, BIOUG34670 View Materials - H02 ( CBG). Ontario, Perth, Murphy`s Point Provincial Park , 44.7812 -76.2336, 143 masl, CBG Collections Staff leg. 23. V GoogleMaps .2014, 1 female, BOLD, BIOUG34683 View Materials - F08 ; 1 male, BOLD, BIOUG34694 View Materials - D02 ( CBG). GoogleMaps Ontario, Warsaw, Ferris Provincial Park, 44.2829 -77.7963, 131 masl, CBG Collections Staff leg. 12.IX.2014, 1 female, BOLD, BIOUG 34924- D04 ( CBG). GoogleMaps New Brunswick, Springfield , Belleisle Regional High School , 45.669 -65.836, 13 masl, S. Lawson leg. 2.X.2015, 1 male, BOLD, BIOUG25544 View Materials - C01 GoogleMaps ; 1 female, BOLD, BIOUG25544 View Materials - F11 ( CBG). Prince Edward Island, Prince Edward Island National Park , 46.4123 -63.085, 6 masl, P. Ayles leg. 26. VI GoogleMaps .2013, 1 male, BOLD, BIOUG10366 View Materials - H09 ; 1 female, BOLD, BIOUG10368 View Materials - A04 ; 10.VII.2013, 1 male, BOLD, BIOUG10435 View Materials - C08 ; 5. VI .2013 , 1 male, BOLD, BIOUG10660 View Materials - D02 ; 1 male, BOLD, BIOUG10787 View Materials - B11 ( CBG). GoogleMaps British Columbia, Kootenay National Park, Kootenay Pond, 50.892 -116.041, 1188 masl, BIObus 2014 leg. 14.VIII.2014, 1 female pupa, BOLD, BIOUG22761 View Materials - H04 ( CBG). GoogleMaps NWT, Nahanni National Park Reserve , Nailicho ( Virginia Falls ), 61.606 -125.758, 578 masl, Parks GoogleMaps Canada leg. 27. VI .2014, 1 male, BOLD, BIOUG16983 View Materials - G08 ; 12.VII.2014, 1 female, BOLD, BIOUG17213 View Materials - H03 ( CBG). NWT, Reindeer Depot, Mackenzie delta, 68°42’N 134°07’W, J. R GoogleMaps . Vockeroth leg. 5.VII.1948, 7 males (5 pinned, 2 on slide) 1 female on slide; 15.VII.1948, 2 females pinned; 1.VIII.1948, 1 female pinned ( CNC) .
USA. Alaska, Big Delta, Boleo Lk., 64.14 -145.81, W. R . M. Mason leg. 22. V .1955 , 1 male on slide, 32 ( CNC). Alaska, pond near Kuzitrin River , 65.396 -164.496, D. J. Taylor leg. 31.VII.2013, 6 larvae in EtOH, Kuzitrin 4 ( UBB) GoogleMaps . Alaska, pond near Nome Airport , 64.514 -165.422, D. J. Taylor leg. 3.VIII.2011, 4 larvae in EtOH, Nome 1 2011 ( UBB) GoogleMaps . North Carolina, Highlands , 35°03ʹN 83°12ʹW, 3800’, J. R GoogleMaps . Vockeroth leg. 24. VI .1957 , 4 males (1 on slide, 3 pinned, 1 hypopygium in microvial); 8. VI .1957 , 1 female pinned; 10. VI .1957 , 1 female pinned; 21. VI .1957 , 1 male pinned ( CNC).
Redescription. Adult male. Head light to dark brown, bearing pale setae. Non-setose area of occiput brownish. Clypeus bearing dark setae. Penultimate flagellomere 285 (271–313), apical flagellomere 204 (163–228), penultimate/apical 1.42 (1.21–1.78, n=6). Lengths of palpal segments 2–5: 105 (89–113), 208 (177–234), 181 (172–194), 336 (267–393, n=6). Thorax. Scutellum and mediotergite orange–dark brown ( Fig. 11 View FIGURE 11 ). Coloration of pleuron composed of pale and dark areas: most of the katepisternum darkened; antepronotal lobe, postpronotum, anepimeron, part of metanepisternum and part of anepisternum slightly darkened, halteres whitish. Thoracic setae (n=6): antepronotal lobe 33 (27–45), postpronotal 4 (3–6), proepisternal 5 (4–7), katepisternal 6 (6–7), anepisternal 16 (12–19), anepimeron 8 (7–9), supra–alar 2 (1–2, n=3). Legs pale yellow–light brown. Foreleg, lengths of fe, ti and ta1–ta5 (n=6, except t4 and t5 n=5): fe 1708 (1667–1750), ti 1773 1722–1859), t1 825 (773–864), t2 499 (455–534), t3 397 (344–432), t4 271 (230–291), t5 196 (177–221). Midleg, lengths of fe, ti and ta1–ta5 (n=6): fe 1467 (1360–1556), ti 1413 (1314–1472), t1 689 (619–727), t2 398 (348–443), t3 318 (261–364), t4 218 (188–235), t5 179 (160–193). Hind leg, lengths of fe, ti and ta1–ta5 (n=6, except fe and t4 n=5): fe 1839 (1723–1945), ti 1761 (1639–1933), t1 1012 (869–1114), t2 581 (454–643), t3 404 (318–443), t4 248 (209–271), t5 185 (171–197). Wing (n=7). Length 3485 (2777–3831), width 770 (643–882), length/width 4.52 (4.32–4.73); fork of R 2+3 402 (318–448), fork of M 1+2 370 (306–424), R 3 1054 (888–1205), M 1 903 (782–979), number of setae on squama 30 (24–39, n=5). Abdomen. Tergal pattern either with dark specks on pale ground or tergites almost uniformly brown ( Fig. 5 View FIGURE 5 c–e); in former case tergites 2–6 with central and lateral specks that are not connected. Hypopygium ( Fig. 11 View FIGURE 11 ). Gonocoxite brown, length 484 (455–546), width 141 (118–193), length/width 3.47 (2.59–3.91, n=13). Gonostylus brown, somewhat darker than or about as dark as gonocoxite, rather narrow, length 398 (345–432), width 32 (25–39), length/width 12.55 (10.21–13.93, n=14). Paramere ( Fig. 11 View FIGURE 11 b–e) almost unicolorous, brownish, gently curved medially, apical claw stout and curved; length 132 (122–152, n=13).
Adult female. In general similar to male. Penultimate flagellomere 165 (157–175), apical flagellomere 172 (159–186), ratio penultimate/apical 0.96 (0.91–1.04) (n=4). Lengths of palpal segments 2–5 (n=4 except 5 th segment n=3): 99 (79–115), 217 (195–240), 193 (171–209), 345 (324–376). Thoracic setae (n=2): antepronotal lobe 28–34, postpronotal 7–8, proepisternal 5–9, katepisternal 4–11, anepisternal 13–14, anepimeron 5–14, supra–alar 1–3. Wing length 3587 (3025–4337), width 964 (864–1155), length/width 3.72 (3.43–4.20) (n=5); fork of R 2+3 372 (134–483), fork of M 1+2 330 (257–402), R 3 1292 (1090–1460), M1 1111 (772–1318) (n=4), number of setae on squama 35–47 (n=2). Foreleg, lengths of fe, ti and ta1–ta5 (n=4): fe 1621 (1374–1747), ti 1691 (1397–1856), ta1 790 (717–871), ta2 459 (379–509), ta3 374 (322–407), ta4 257 (202–292), ta5 188 (128–231). Midleg, lengths of fe, ti and ta1–ta5 (n=4 except ta5 n=3): fe 1477 (1301–1643), ti 1352 (1130–1489), ta1 647 (520–736), ta2 365 (255–437), ta3 270 (217–326), ta4 206 (147–240), ta5 188 (175–204). Hind leg, lengths of fe, ti and ta1–ta5 (n=2): fe 1779–1950, ti 1703–1950, ta1 978–1139, t2 542–667, t3 390–433, t4 244–284, t5 185–193. Dark specks of abdominal tergites, if present, blurred, not as clear as in males Fig. 5d View FIGURE 5 ).
Pupa. Thoracic respiratory organ slender, subapically constricted ( Fig. 9c View FIGURE 9 ), length 891 (693–1057), width 220 (170–284), length/width 4.09 (3.31– 4.92 n =26). Lateral ribs of terminal processes pale–light brown, mid rib light brown–dark brown.
IV instar larva. Anal fan setae 22 (18–25, n=17). Anal hook pale – brown in color. Mandibular teeth 1–4 darkened in their apical halves. Mandibular fan bristles 17.1 (14–23, n=33). Number of lateral teeth 5.4 (5–6, n=23); uppermost lateral tooth about as long as or longer than subordinate tooth ( Fig. 10d View FIGURE 10 ). Labral blade elongated, moderately serrated or almost smooth ( Fig. 10e View FIGURE 10 ), length 268 (228–299), width 49 (39–62), length/width 5.49 (4.38–6.85, n=24). Length of antenna 536 (443–628, n=25).
DNA barcoding. Chaoborus albipes was very divergent from other members of the complex (>17%) ( Table 1). Intraspecific variation was the greatest within the species complex ( Table 1), but as with C. flavicans , this divergence was mainly due to the presence of differentiated geographic clades ( Fig. 16 View FIGURE 16 ). Again, these shallow geographic clades presumably resulted from survival in separate Pleistocene glacial refugia. The pattern is not merely an isolation by distance effect as single clades are almost identical over thousands of kilometers (e.g., Nome [Alaska], Kodiak [Alaska], and British Columbia). Interestingly, two Western Nearctic/Beringian clades may presently overlap in range. The probability of identification from barcoding information alone was very high (>98%). Barcoding sequences of Alaskan specimens from the present study were>99.6% similar to the shorter Alaskan sequences of C. cf. flavicans of Ballinger et al. (2017). Chaoborus albipes includes three BIN clusters in BOLD: BOLD:ADT7561 ( Finland), BOLD:ADT7894 (Northwest Territories, Central Russia) and BOLD:AAM6295 (eastern Nearctic). The two latter BINs were included in Hebert et al. (2016), a study that estimated the species richness of Canadian insects. Because C. albipes is composed of two Nearctic BIN clusters, it is possible that there are other phantom midge species with more than one BIN. Hence, the presence of 19 chaoborid species, in contrast to the 12 currently known, in Canada is perhaps an overestimation.
Comments. Chaoborus albipes is a widespread and rather common Holarctic species that has been hitherto confused with C. flavicans . The species, however, is distinguished from C. flavicans on morphological, molecular and ecological grounds. The paramere of adult males has a characteristic apical claw, that is curved and stout in structure.Also, below the apical claw is a large and protruding “lower lip” that is modest in C. flavicans and C. posio sp. n. The pupa is similar to C. flavicans (see above) and the larva is similar to C. posio sp. n. (see below).
The original description of the species ( Johannsen 1903) is quite superficial and is based on an adult female. The holotype female was perhaps pinned and a wing was slide mounted; presently the slide remains, but the rest of the specimen is lost (J.J. Dombroskie pers. comm.). The female holotype was collected from the eastern USA, Ithaca, New York. Chaoborus albipes specimens from eastern North America (e.g. Nova Scotia, New Brunswick, Ontario, and Prince Edward Island) have a characteristic pattern on the tergites. Unlike C. flavicans , there are no transverse dark subapical bands. Instead, C. albipes has have separate lateral and median specks on the tergites 2–6 ( Fig. 5c,d View FIGURE 5 ). The original description states, “… lateral margin sparsely sprinkled with small irregular black specks.” ( Johannsen 1903, p. 398). Thus, despite the female sex of the holotype and a partially lost specimen, it is concluded that C. albipes is the oldest available name for this taxon. Chaoborus albipes was redescribed by Felt (1904) and Richardson (1912) soon after its initial description; the tergal pattern in both publications is consistent with the current concept of C. albipes . Lateral specks may also be partly due to the dispersion of chromatophores from the larval air sacs to the tissues of adult flies ( Borkent & Borkent 2008), but it is evident that the abdominal patterns in C. albipes (especially eastern Nearctic populations) and C. flavicans are specific ( Fig. 5 View FIGURE 5 ). Sayomyia rotundifolia is treated as a new junior synonym of C. albipes . The lectotype male of S. rotundifolia , as designated here, consists of a slide mounted abdomen ( Fig. 2a,b,d View FIGURE 2 ). The hypopygium of the lectotype is in moderately good condition and the shape of the paramere can be seen from the slide ( Fig. 2b View FIGURE 2 ), despite being somewhat pressed.
In the original description of Sayomyia rotundifolia , the larva, pupa, adult female and male are described ( Felt 1904). The description of the larva is either erroneous or based on the III instar larva, because, according to Felt (1904, p. 368) there are four pairs of post-antennal filaments, the anal fan (as “ventral tuft”) consists of 16 setae and the number of mandibular fan bristles is seven. Nonetheless, the paralectotype larva is a IV instar larva with 10 postantennal filaments, 21 anal fan and 12 mandibular fan bristles. Felt (1904) implicitly considers the features given by him as diagnostic differences between S. albipes and S. rotundifolia , a diagnosis later repeated by Johannsen (1934). A further confusion with the paralectotype larvae is its taxonomic identity. That specimen is slide-mounted as a whole, but fortunately the mandibles and labral blades can be clearly seen. The paralectotype larva ( Fig. 2c View FIGURE 2 ) has four mandibular lateral teeth, and the uppermost of these is somewhat smaller than the subordinate tooth. This character and the low number of mandibular fan bristles (12) taken into account, the paralectotype actually fits the concept of C. flavicans better than that of C. albipes .
Chaoborus albipes is a widespread species with notable intraspecific variation. This variation is evident in mitochondrial and nuclear DNA ( Dupuis et al. 2008, see above) and in the coloration of adult specimens ( Fig. 12 View FIGURE 12 ). Adult specimens in temperate North America and Japan have light-orange brown or brown scutellar stripes and pleural markings; abdominal tergites are whitish in ground color with modest medial and lateral dark markings (spots, specks) ( Fig. 12b,d,f View FIGURE 12 ). Specimens from subarctic North America, Hokkaido and Fennoscandia have dark thoracic markings and brown tergites ( Fig. 12a,c,e View FIGURE 12 ). The parameres, which are essential characters in the identification of species based on adult males, hardly varies. Despite its small size and apparent simplicity, it is rather complex in structure. The outline of the paramere depends on the angle to the observer ( Fig. 11 View FIGURE 11 b-d). The differing positions of the character on the slide or petri dish (see below) can be confused with variation. Intraspecific variation in larval and pupal characters is currently not well known due to the scarcity of available material. The number of mandibular fan bristles is variable among the studied material, ranging from 14 to 23. However, this variation seems to be population specific. For example, in Joroinen, Savukoski and Kuzitrin, the average numbers are 20.8 (20–23, n=4), 15.3 (14–16, n=7) and 15.3 (14–17, n=6) respectively.
In his revision of North American chaoborids, Cook (1956) redescribed larvae, pupae, adult males and females of C. flavicans . However, it is now clear that Cook mixed two species under the name C. flavicans . This can be judged from the material studied by him and reading the descriptions. For example, specimens from MacKenzie Delta, Reindeer Depot are deposited at the CNC and belong to C. albipes . On the other hand, the description of the tergal color pattern fits C. flavicans . Cook (1956, p. 23) admits that variation in C. flavicans is notable in coloration and “… especially in the genitalic structures of the males. The variation in the penis valves of the males is quite marked, so that if only the extremes were available it might seem that two distinct species are involved.” Cook states that there is a continuum between the extremes and it is impossible to segregate individual specimens. In retrospect it has to be agreed that the parameres figured by Cook actually depict intraspecific variation; not in C. flavicans , but in C. albipes . Material studied by Cook was rather extensive, covering ca. 180 specimens (all life stages combined). In this study JS has examined> 100 males of C. albipes and> 100 males of C. flavicans , and in all cases identification based on the morphology of parameres has been unambiguous.
Distribution. Holarctic. Probably very widespread and common in North America, from subarctic to temperate vegetation zones. In the Palaearctic known from north boreal to south temperate ecoregions; perhaps absent from Central Europe. In this study verified from USA (Alaska, New York, North Carolina), Canada (North West Territories, British Columbia, Ontario, Nova Scotia, New Brunswick, Prince Edward Island), Norway (Buskerud), Finland (north boreal, south boreal), Russia (South Urals, Far East), and Japan (Hokkaido, Sado Island, Honshu, Shikoku). Also known from Massachusetts ( Richardson 1912), New Jersey ( Ogawa 2007), Wisconsin ( Dickinson 1944), Alaska, Indiana ( USA) and Alberta ( Canada) ( Dupuis et al. 2008; Ballinger et al. 2014; 2017).
Ecology. In Finland the species is uni- or bivoltine, pupation and emergence of overwintered larvae occur in early summer (only reared material were hitherto available). In a pond in northern Finland, pupae of the overwintering generation were collected in early June, and two months later, in early August, pupal exuviae and first and second instar larvae were observed. This indicates a bivoltine lifecycle or a very long pupation time of the overwintered generation. Habitats are mostly small but permanent fishless ponds, from ca. 20 m 2 to 2700 m 2 in area, and one to a few meters in depth. Ponds are often influenced by humic substances, i.e. water color is brownish, most often with a mire margin (bog or fen vegetation). Larvae occur in sympatry usually with C. obscuripes and seldom with C. nyblaei , C. flavicans and C. posio sp. n.. The only known collection site in Norway is near a small lake 495 m above sea level, populated by fish ( Perca fluviatilis and Salmo trutta , K-M Olsen, pers.comm.). However, it is not known whether the specimens in the Malaise trap sample originated from this lake or fishless ponds nearby. Remarkably, one Finnish population is known from a boreal humic pond (area 6900 m 2, max depth 8 m) that is inhabited by a fish ( P. fluviatilis, J. Salmela , pers.obs.). It is not yet known if the larvae of this population perform diel vertical migration. In North America, C. albipes is known from small forest ponds ( Felt 1904, Richardson 1912) and from humic tundra pools in Alaska ( Taylor et al. 2015), where the species may coexist in deeper ponds with C. americanus . In Japan it is known from north to south, and is possibly bivoltine (adults collected in May and late August). According to Richardson (1912), larvae eat unicellular organisms and Copepoda. Alaskan larvae do not perform diel vertical migrations in shallow ponds ( Dupuis et al. 2008), but it is not known if the larvae can withstand higher pressure of deeper waters. The RNA virus family, Phasmaviridae was first described from C. albipes and C. trivittatus (Loew) in the Nome region of Alaska ( Ballinger et al. 2014). This family of viruses is now known from most major insect groups and is perhaps maternally inherited in Culicomorpha. Notably phasmaviruses have a high prevalence in larval stages, but little is known of the fitness effects on the host.
Based on indirect evidence it is assumed that C. albipes is more common in the Nearctic than in the Palaearctic. First of all, despite the long entomological tradition dating back to 18 th century in Europe, the species was first detected from North America. Secondly, C. albipes (51) and C. flavicans (345) are both well represented among the DNA barcoded specimens from North America (BOLD public data base, accessed 20.VIII.2020), whereas there are only a few barcoded C. albipes specimens from the Palaearctic (only one, if the Finnish and Russian specimens that were purposely analyzed are excluded). Finally, it is likely that C. albipes prefers shallow (<2 m deep), fishless ponds ( Felt 1904; Richardson 1912; Cook 1956 in part; Dupuis et al. 2008; Taylor et al. 2015; material studied here). In central and southern Europe C. crystallinus is a common and abundant Chaoborus species in such habitats (e.g. Kuper & Verberk 2011; Arranz et al. 2015). In contrast, C. crystallinus is not that widespread in the North America, being absent from eastern North America ( Borkent 1981) where C. albipes seems to be common (see material examined above). It is possible, that the favored habitat of C. albipes is largely occupied and dominated by C. crystallinus in Europe and the former is either absent or extremely rare there due to competition. However, the species is likely rather common yet overlooked in Fennoscandia. Up to June 2020, the species was known from 22 locations in Finland and Norway, of which 68 % were found during April–June 2020.
| CUI |
Central College, Iowa |
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
| CBG |
Australian National Botanic Gardens, specimens pre-1993 |
| NMNS |
National Museum of Natural Science |
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chaoborus albipes ( Johannsen, 1903 )
| Salmela, Jukka, Härmä, Oskari & Taylor, Derek J. 2021 |
Chaoborus (Chaoborus) rotundifolia:
| Hennig, W. 1968: 74 |
Chaoborus (Chaoborus) flavicans: Cook 1956: 23
| Cook, E. F. 1956: 23 |
Chaoborus albipes: Johannsen 1934: 44
| Belkin, J. N. & Schick, R. X. & Heineman, S. J. 1966: 22 |
| Dickinson, W. E. 1944: 357 |
| Johannsen, O. A. 1934: 44 |
Chaoborus rotundifolia:
| Johannsen, O. A. 1934: 43 |
Chaoborus crystallinus: Yamada 1932: 230
| Matheson, R. 1944: 95 |
| Yamada, S. 1932: 230 |
Chaoborus crystallina: Matheson 1925: 159
| Matheson, R. 1925: 159 |
Chaoborus (Chaoborus) albipes:
| Hennig, W. 1968: 74 |
| Dyar, H. G. & Shannon, R. C. 1924: 211 |
Sayomyia albipes: Felt 1904: 363
| Felt, E. P. 1905: 497 |
| Dyar, H. G. 1905: 16 |
| Felt, E. P. 1904: 363 |
Sayomyia rotundifolia
| Felt, E. P. 1905: 497 |
| Dyar, H. G. 1905: 16 |
| Felt, E. P. 1904: 366 |
Corethra albipes
| Richardson, C. H. 1912: 202 |
| Johannsen, O. A. 1903: 398 |