Orthochromis indermauri, Schedel & Vreven & Manda & Abwe & Manda & Schliewen, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4461.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:B646DF09-BADE-43F1-9CE5-39EF1E2971A8 |
|
DOI |
https://doi.org/10.5281/zenodo.5995509 |
|
persistent identifier |
https://treatment.plazi.org/id/5367274E-8107-5171-FF79-D1F1FBE9C6D6 |
|
treatment provided by |
Plazi |
|
scientific name |
Orthochromis indermauri |
| status |
sp. nov. |
Orthochromis indermauri sp. nov.
Orthochromis sp. “Chomba” Indermaur 2014
Holotype. ZSM 46853 (1, ex ZSM 43080, 54.0 mm SL), Zambia, Lufubu River, below last series of rapids near Chomba village, ~ 25.5 km (air distance) from confluence with Lake Tanganyika and 20 km (air distance) south of Sumbu (-8.687010/30.556273)
Paratypes. ZSM 46855 (13, 35.8–68.9 mm SL), Zambia, Lufubu River, Lower Lufubu at Chomba Village, ~ 30 km from confluence with Lake Tanganyika , Northern Province (-8.686376/30.563983). — ZSM 46854 (1, 61.2 mm SL), Zambia, Lufubu River, Lower Lufubu at Chomba Village, ~ 30 km from confluence with Lake Tanganyika , Northern Province (-8.686376/30.563983). — ZSM 43083 (4, 45.6–59.4 mm SL), collected with holotype. — ZSM 43080 (2, 42.0– 43.1 mm SL), collected with holotype. — ZSM 44283 (3, 50.8-63.5 mm SL), Zambia, Lufubu River, Lower Lufubu at Chomba Village, ~ 30 km from confluence with Lake Tanganyika , Northern Province (-8.686376/30.563983). — MRAC 2018-006 View Materials -P-0001-0002 (2, ex ZSM 44283, 56.8– 51.9 mm SL) Zambia, Lufubu River, Lower Lufubu at Chomba village, ~ 30 km from confluence with Lake Tanganyika , Northern Province (-8.686376/30.563983). — MRAC 2018-006 View Materials -P-0003-0008 (6, 43.3–64.1 mm SL), Zambia, Lufubu River, Lower Lufubu at Chomba village, ~ 30 km from confluence with Lake Tanganyika , Northern Province (-8.686376/30.563983).
Diagnosis. Orthochromis indermauri is distinguished from all all species currently placed in Orthochromis (sensu de Vos & Seegers, 1998) except O. torrenticola , by having hypurals 1 and 2 clearly separated or separated by distinct seam (vs. always fused). It is further distinguished from Malagarasi- Orthochromis species, except O. mazimeroensis , O. malagaraziensis , and O. rubrolabialis , by having fewer caudal vertebrae (14–15 vs. 16–18) and total vertebrae (28–29 vs. 30–32). It is also distinguished from O. luichensis , O. malagaraziensis , O. mazimeroensis , O. mosoensis by having more inner series of teeth in upper jaw (3–5 vs. 1–2). Moreover, it differs from O. kasuluensis by having fewer anal-fin rays (7–9 vs. 10); from O. malagarazienisis by having more scales between upper lateral line and dorsal-fin origin (5–7 vs. 3–4) and by having more ceratobranchial gill rakers (8–11 vs. 6–7); from O. mazimeroensis by having more abdominal vertebrae (14–15 vs. 12–13); from O. mosoensis and O. rubrolabialis by having more ceratobranchial gill rakers (8–11 vs. 5–7) and total gill rakers (11–15 vs. 8–10); from O. uvinzae by having fewer horizontal line scales (25–29 vs. 30–32), fewer dorsal-fin spines (17–18 vs. 19– 20) and by position of pterygiophore supporting last dorsal-fin spine (vertebral count: 16–17 vs. 18–19). It is distinguished from O. kalungwishiensis , O. luongoensis , and O. torrenticola by having fewer horizontal line scales (28–29 vs. 30–32) and by having fewer caudal vertebrae (14–15 vs. 17–18). Further, it differs from O. luongoensis and O. machadoi by having fewer series of scales on cheek (0–1 vs. 2–5); from O. kalungwishiensis by having fewer total vertebrae (28–29 vs. 31–33). It is distinguished from S. neodon by having fewer horizontal line scales (28–29 vs. 30–31), more circumpeduncular scales (16 vs. 12), fewer caudal vertebrae (14–15 vs. 16–17), fewer total vertebrae (28–29 vs. 30–32), fewer dorsal-fin rays (8–10 vs. 11–12) and by having hypurals 1 and 2 clearly separated or separated by distinct seam (vs. fused). It differs from H. snoeksi by having fewer scales on cheek (0–1 vs. 2–3), fewer horizontal line scales (25–29 vs. 30–31), more abdominal vertebrae (14–15 vs. 13), fewer caudal vertebrae (14–15 vs. 17), fewer total vertebrae (28–29 vs. 30), more anal-fin rays (7–9 vs. 5–6), more dorsal-fin spines (17–18 vs. 16), more ceratobranchial gill rakers (8–11 vs. 6) and total gill rakers (11–15 vs. 9); from H. bakongo by having more inner series of teeth (3–5 vs. 1–2), more dorsal-fin spines (17–18 vs. 14–15) and in position of pterygiophore supporting last dorsal-fin spine (vertebral count: 16–18 vs. 13–14); from H. moeruensis by having hypurals 1 and 2 clearly separated or separated by distinct seam (vs. always fused). Meristic values of O. indermauri overlap with those of H. vanheusdeni but is distinguished by differences in head mask (e.g. nostril stripe present vs. absent; caudal corner of cheek with blackish element vs. no such element present) and by size and colouration of eggspot-like maculae on anal fin (e.g. deep red centre vs. orange centre in H. vanheusdeni ). It is distinguished from O. mporokoso and O. katumbii by having fewer caudal vertebrae (14–15 vs. 16–17), fewer total vertebrae (28–29 vs. 30–31) and by having hypurals 1 and 2 and hypurals 3 and 4 clearly separated or separated by distinct seam (vs. always fused). Further from O. mporokoso by having fewer series of scales on cheek (0–1 vs. 2– 4); from O. katumbii by having fewer horizontal line scales (25–29 vs. 30–31). It is distinguished from O. kimpala by having fewer series of scales on cheek (0–1 vs. 3–4) and by having more dorsal-fin spines (17–18 vs. 15–16). Meristic values of O. indermauri overlap with those of O. gecki but is distinguished by having a wider interorbital width (13.5–18.2 vs. 9.6–12.9 %HL).
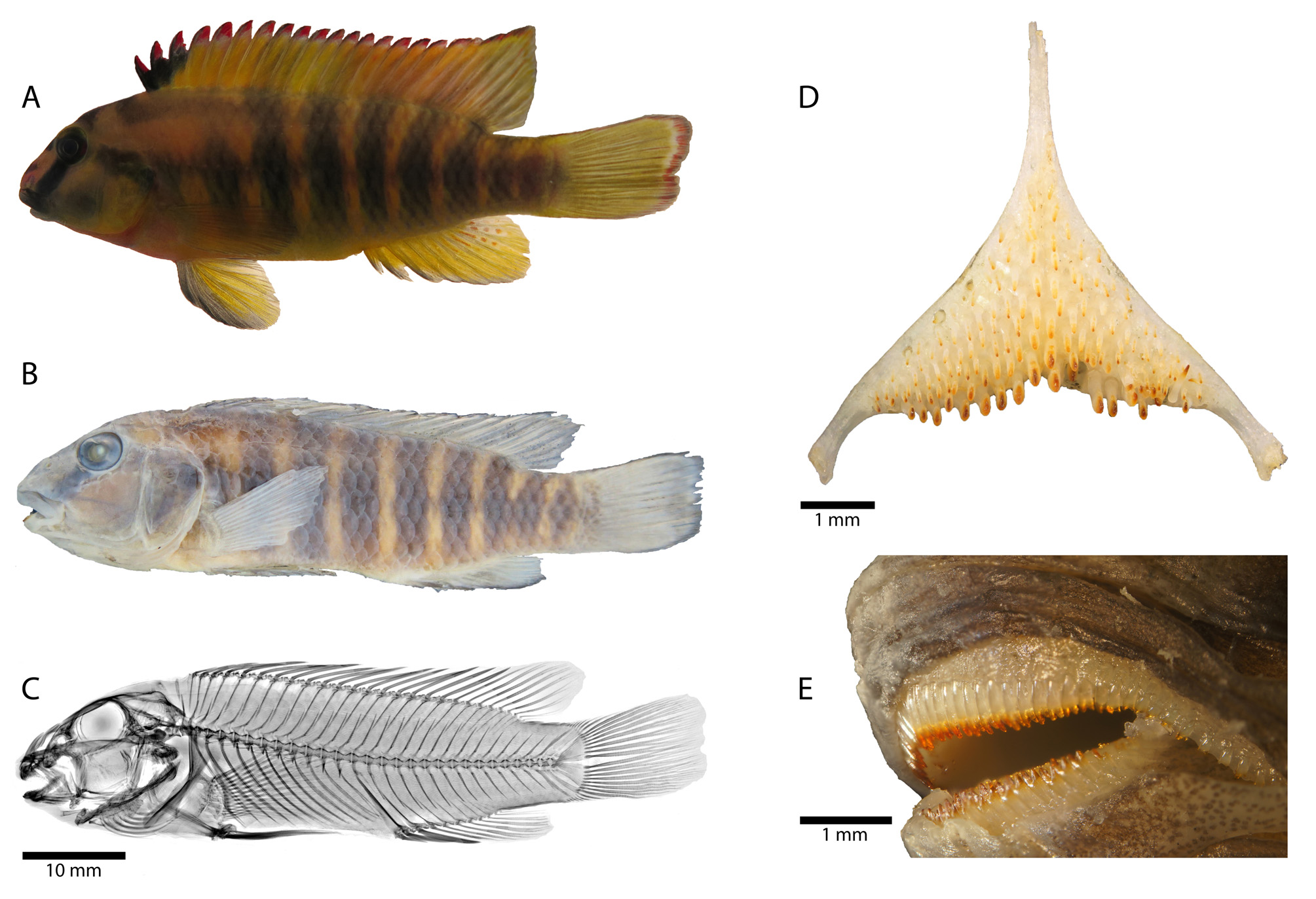
Description. Morphometric measurements and meristic characters are based on 21 out 32 type specimens. Values and their ranges are presented in Table 6. For general appearance see figure 7. Maximum length of wild caught specimens 68.9 mm SL. Moderately slender species with maximum body depth (24.5–29.9 % SL) slightly posterior or at level of first dorsal-fin spine, decreasing rather gradually towards caudal peduncle (but decreasing relatively quick just before caudal peduncle). Caudal peduncle rather short and deep (ratio of caudal peduncle length to depth: 1.2–1.4). Head length almost one third of standard length. Dorsal-head profile moderately curved without prominent nuchal gibbosity. Eye diameter always larger than interorbital width. Jaws slightly retrognathouswith lower jaw shorter than upper jaw. Posterior tip of maxilla not reaching anterior margin of orbit but slightly before. Lips not noticeably enlarged or thickened. Two separate lateral lines.
Squamation. Flank above and below lateral lines covered with cycloid scales, even in smaller specimens. Belly and chest covered by deeply embedded minute scales giving appearance of being scaleless. Ventro-anterior area of pectoral fin with small, deeply embedded cycloid scales. Chest to flank transition with small, embedded cycloid scales.
Snout scaleless. Interorbital region with minute, deeply embedded cycloid scales. Nape and occipital region covered with minute to small, embedded cycloid scales becoming slightly larger towards occipital region. Cheek appears scaleless, but rarely small deeply embedded cycloid scales present just below eye; 0–1 scale rows on cheek. Cycloid scales on operculum of variable size (small to medium) and mainly of circular shape; opercular blotch only on anterior margin covered by medium sized scales, main area of opercular blotch scaleless. 5–7 scales on horizontal line from edge of postero-dorsal angle of operculum to anterior edge of operculum.
Upper lateral line scales 20–23 and lower lateral line 7–11. Horizontal line scales 27–29. Caudal fin with 0–2 pored scales. Upper and lower lateral lines separated by two scales. 3–5 scales between upper lateral line and dorsal-fin origin. Anterior part of caudal fin covered with 2–3 vertical rows of small cycloid scales with median scales being slightly larger; scaled area of caudal fin extended posteriorly with interradial scales (approximately up to two thirds of caudal fin). Sixteen scales around caudal peduncle.
Jaws and dentition. Anterior teeth of outer row of upper and lower jaw bicuspid to subequally bicuspid, large and very densely set; teeth smaller towards corner of mouth, more widely set and becoming unicuspid (rarely tricuspid or subequally bicuspid teeth present on upper jaw near corner mouth). Individual bicuspid teeth with not expanded brownish crown, cusps (tips pointed) slightly compressed and narrowly set, and neck slender. Outer row of upper jaw with 42–59 teeth and outer row of lower jaw with 26–45 teeth (specimens: 35.8–68.9 mm SL); larger specimens generally with more teeth. Inner upper jaw with 3–5 tooth rows and 3–4 rows (rarely 2) in lower jaw, all with small tricuspid teeth.
Lower pharyngeal bone ( Fig. 7 View FIGURE 7 ) of single dissected paratype (ZSM 46854, 61.2 mm SL) about as wide as long with anterior keel about 0.6 times length of dentigerous area. Dentigerous area of lower pharyngeal bone about 1.5 times wider than long, with 11+11 teeth (empty tooth-sockets included) along posterior margin and eight teeth along midline. Anterior pharyngeal teeth (towards keel) bevelled and slender; teeth posterior row larger than anterior ones, bevelled (bicuspid; well-developed major and minor cusp). Largest teeth medially in posterior row. Teeth along midline slightly larger than more lateral ones.
......continued on the next page
Gill rakers. Total gill raker count 11–15, with 2–4 epibranchial, one angle, and 8–10 ceratobranchial gill rakers. Anteriormost ceratobranchial gill rakers smallest increasing in size towards cartilaginous plug (angle). Anterior gill rakers on ceratobranchial generally unifid, sometimes bifid towards cartilaginous plug. Gill raker on cartilaginous plug shorter than longest ceratobranchial gill raker and epibranchial gill rakers further decreasing in size.
Fins. Dorsal fin with 17–18 spines and with 8–10 rays. First dorsal-fin spine always shortest. Dorsal-fin base length between 56.9–65.4 % SL. Posterior end of dorsal-fin rays extending slightly beyond caudal fin base; posterior tip of anal fin reaching slightly before or at caudal fin base. Caudal fin outline subtruncate and composed of 27–29 rays (16 principal caudal-fin rays and 11–13 procurrent caudal-fin rays). Anal fin with 3 spines (3rd spine longest) and 7–9 rays. Anal-fin base length between 16.7–21.9 % SL. Pectoral fin with 14 to 15 rays and length between 19.7–25.6 % SL; longest pectoral ray not reaching or in rare cases almost reaching level of anus (ending approximately 1-2 flank scale widths in front of it). First upper and lower pectoral-fin rays very short to short. Pelvic fin with 1 st spine thickly covered with skin and 5 rays. Pelvic fin base at same level pectoral fin base. Longest pelvic-fin ray not reaching or in rare cases almost reaching anus (ending approximately 1-2 flank scale widths in front of it).
Vertebrae and caudal fin skeleton. 28–29 total vertebrae (excluding urostyle element), with 14–15 abdominal and 14–15 caudal vertebrae. Pterygiophore supporting last dorsal-fin spine inserted between neural spines of 16th and 17th or 17th and 18th vertebra (counted from anterior to posterior). Pterygiophore supporting last anal-fin spine inserted between haemal spines of 15th and 16th or 16th and 17th vertebra, rarely between rips of 14th and haemal spine of 15th vertebra (N=1). Single predorsal bone (=Supraneural bone) present. Hypurals 1 and 2 either clearly separated or separated by distinct seam but never fused into single seamless unit. Hypurals 3 and 4 either fused into single seamless unit or separated by distinct seam.
Colouration in life (based on field photographs of adult specimens). Body ground colouration brownish yellow, towards belly more yellowish; dorsum brownish yellow to pale brown; chest below pectoral fins yellow becoming reddish ventrally; belly yellow. Dorsal head surface pale brown dorsally with reddish speckles; ethmoidal area pale brown and densely speckled with reddish spots, especially in dominant males ( Indermaur 2014). Iris reddish posteriorly, yellow dorsally, remaining greyish. Upper lip dark grey anteriorly sometimes with reddish speckles; lower lip light greyish, yellowish posteriorly. Cheek pale brown becoming yellowish towards corner mouth and mental area; blackish pear-shaped blotch at caudal-ventral corner, expanding to anterior extension of midlateral band. Branchiostegal membrane along operculum yellow becoming whitish to pale pinkish ventrally. Operculum yellow with black opercular spot, which is fused with anterior extension of midlateral band which is ending just anterior of the eye. Broad blackish lachrymal stripe between orbit and caudal corner of upper lip. A relatively faint greyish nostril stripe, sometimes covered by many reddish speckles. Relatively wide blackish interorbital stripe. Blackish supraorbital stripe connected with nape band. Nape band ending slightly anterior of dorsal-fin origin and fused with dorso-lateral band. Dorso-lateral band slightly below dorsal fin base and visible up to third or fourth anterior vertical bar. Relatively thin midlateral band ending with dark blotch just posterior base caudal fin. 7–9 blackish vertical bars crossing midlateral band and extending onto dorsal fin almost to fin margin; anterior-most vertical bar (just behind operculum) less intensive than remaining bars. Vertical bars wider than space between them. Dorsal-fin membrane yellow without maculae, skin/membrane of first three dorsal-fin spines black creating the appearance of a broad black oblique band between 1 st and 4th spine. Margin of spiny part dorsal fin with fine black outline and red (distally) and transparent submarginal band; rayed part of dorsal fin lacks transparent submarginal band. Anal fin yellow; margin greyish outlined. Posterior half of anal fin with deep-red maculae on membrane (between last four anal-fin rays); maculae elongated proximally becoming more rounded distally (maculae not to fin margin but ending slightly before). In general, these maculae resemble egg-spots: large deep red centre surrounded by faint greyish ring then by ill-defined transparent ring. Caudal fin yellow with deep red maculae as described for anal fin but only with roundish maculae. Caudal fin with reddish marginal band with narrower bluish submarginal band; another submarginal band of red maculae (intensity varies). Outer caudal-fin rays outlined in black. Pectoral fin yellow to orange. Pelvic fin yellowish with dark greyish anterior margin spanning spine and first two rays.
Juvenile colouration in life. (based on tank-raised juveniles of approximately 20 to 30 mm SL; Fig. 9 View FIGURE 9 ). Ground colouration greyish to beige. Patterns and head mask as described for adults. No reddish speckles present. Dorsal and midlateral band, greyish vertical bars on flank as described for adults. Dorsal fin hyaline to beige with vertical flank bars extending onto fin. Anal, caudal, pectoral and pelvic fin hyaline.
Colouration in alcohol. Colouration and melanin patterns similar to live specimens, but due the preservation procedure of specimens, i.e., first formalin fixation, transfer to 75 % EtOH etc., specimens tend to lose original colouration (especially melanin patterns more intense than in live specimens). Overall body ground colouration pale brownish to pale yellowish; chest and belly beige to yellowish. Branchiostegal membrane greyish-beige to greyish-brown. Dorsal head surface pale brownish; ethmoidal region greyish-brown. Upper lip brownish and lower lip beige. Cheeks beige to brownish; pear-shaped blotch on lower caudal corner of cheek greyish-brownish and less prominent than in living specimens. Operculum greyish and with opercular spot as described above. Head mask and mid- and dorso-lateral band and vertical bars brownish to greyish. Dorsal fin light greyish except dark grey skin/membrane of first three anterior spines, remaining fin with black margin; extensions of vertical bars on dorsal fin dark grey. Anal fin light greyish; margin outlined in dark grey; no maculae visible. Caudal fin light greyish and margins outlined in black; some thin blackish streaks on membrane between rays may be present. Pectoral fin light grey. Pelvic fin light grey, skin/membrane of pectoral spine and first two rays greyish.
Distribution and biology. Orthochromis indermauri is only known from the lower reaches of the Lufubu River ( Zambia), the third largest tributary of Lake Tanganyika ( Fig. 1 View FIGURE 1 ). Several cascades and waterfalls seem to represent insurmountable barriers to the upstream movement of the lake ichthyofauna hence the fish communities of the upper and lower reaches are clearly distinct. The Upper Lufubu has faunistic similarities to the Congo and Zambezi systems while the Lower Lufubu shows faunistic influences of Lake Tanganyika ( Koblmüller et al. 2012). At the type locality the Lufubu River is rocky with some patches of sand and gravel, about 20 meters wide and on average 50 cm deep ( Fig. 8 View FIGURE 8 ). The water temperature varies throughout the year, 23 °C was measured in July and 28.1 °C in November, the pH ranged between 8.0–8.55, and electrical conductivity around 29 µS ( Indermaur 2014, pers. com. Bernd Egger). O. indermauri is benthic-rheophilic and prefers stretches of fast flowing water where it is found between and among large rocks or patches of gravel. No stomach contents were examined, however, underwater observations indicate it scrapes aufwuchs from the substrate and forages between rocks and patches of sand and gravel ( Indermaur 2014, pers. obs. FS). Orthochromis indermauri is a maternal mouthbrooder. Females in captivity have comparatively small clutches of between 17 and 21 fry (two spawns, pers. com. Adrian Indermaur).
Etymology. The species name indermauri honours the Swiss ichthyologist Dr. Adrian Indermaur, who was the first to document this new species with underwater photographs, videos, and with aquarium observations, thereby contributing to a large extent to our knowledge of behavior and ecology of this species.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |