Pachychilus sp.
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2010.00670.x |
|
persistent identifier |
https://treatment.plazi.org/id/526D476A-FFAF-FFB8-DBC3-0884FBA0F303 |
|
treatment provided by |
Valdenar |
|
scientific name |
Pachychilus sp. |
| status |
|
Pachychilus sp. AY010524 View Materials * HM003671 View Materials *
Cleopatra johnstoni AY 456590
(Smith, 1893)
Lavigeria grandis AY 958771
(Smith, 1881)
Lavigeria sp. A AY958773 View Materials
Paludomus siamensis AY 456614 HM003670 View Materials Blanford, 1903
Tanganyicia rufofilosa AY 456634
(Smith, 1880)
Tiphobia horei Smith, AY 456636
1880
Elimia livescens DQ 311116 DQ311127 View Materials (Menke, 1830)
Elimia interrupta AY 010521* HM003677 View Materials * (Haldeman, 1840)
Pleurocera acuta AF 100994
Rafinesque, 1831
Pleurocera vestita HM 003678 (Conrad, 1834)
Pleurocera canaliculata AF 100991* DQ256747 View Materials (Say, 1821)
Hua jacqueti FJ 471494 HM003679 View Materials (Dautzenberg &
Fischer, 1906)
Juga nigrina (Lea, 1856) AY 010523*
Juga silicula ( Gould, DQ311121 View Materials DQ311135 View Materials 1847)
Abbott (1955), Simone (2001).
Köhler & Glaubrecht (2001). Simultaneous analyses: Brotia pagodula (morphological) + Brotia sp. (molecular)
As Paracrostoma paludiformis (Yen, 1939)
Houbrick (1991b).
Extended analysis only. Sequenced specimen from Madagascar (ZMB 200.287); see also Köhler et al. (2004) [as Melanatria fluminea (Gmelin, 1791) ]
Simone (2001). Midgut for Pachychilus indiorum (Morelet, 1849) (Strong, in press)
Kohnert & Storch (1984a, b), E. E. Strong (unpubl. data). Chromosome number for Cleopatra bulimoides (Olivier, 1804) ( Thiriot-Quiévreux, 2003)
Extended analysis only
Strong & Glaubrecht (2007). Male reproductive anatomy for Lavigeria sp. B ( Michel, 2004) . Sperm ultrastructure for Lavigeria sp. (J. M. Healy & M. Glaubrecht, unpubl. data).
E. E. Strong (unpubl. data). Chromosome number for Paludomus tanschaurica Gmelin, 1791 ( Patterson, 1969) . Sequenced specimen from Thailand (ZMB 200.234); see also Wilson et al. (2004).
Strong & Glaubrecht (2002).
Glaubrecht & Strong (2004), Strong & Glaubrecht (2007).
Jewell (1931), Dazo (1965), Strong (2005). Sperm ultrastructure for Elimia proxima (Say, 1825) ( Bergstrom, Henley & Costello, 1973; Henley, 1973).
Dazo (1965), Strong (2005). Radular morphology for Pleurocera spp. ( Sides, 2005) .
Sequenced specimen from Alabama, USA (NCSM-P-4691); see also Holznagel & Lydeard (2000).
Strong & Köhler (2009). 28S sequence from F. Köhler (AM). Sequenced specimen from Vietnam (ZMB 114.163); see also Strong & Köhler (2009).
Strong & Frest (2007), T. J. Frest (unpubl. data). Chromosome number for Juga hemphilli (Henderson, 1935) ( Thiriot-Quiévreux, 2003)
The primary terminal is indicated in the far left column; supplementary sources of anatomical information and strategies for merging morphological and molecular terminals are detailed under Sources. Unless otherwise indicated, stomach data are based on Strong (in press); chromosome numbers are from Nishikawa (1962), Patterson (1969), Thiriot-Quiévreux (2003), and references therein. Polymorphic coding was used when morphological variation was evident within concatenated terminals. Entries in the 16S and 28S columns are GenBank accession numbers. For 16S sequences, * indicates data originally included in the analysis of Lydeard et al. (2002); newly generated 28S sequences (GenBank accession numbers HM003647 View Materials - HM003679 View Materials ) also indicated with * are for the same specimen (see Lydeard et al., 2002, for details). Voucher and locality information for other newly generated sequences are provided under Sources.
AM, Australian Museum, Sydney; NSCM, North Carolina State Museum of Natural Sciences; ZMB, Berlin Museum of Natural History .
generally regarded as plesiomorphic traits, and are not unique in these regards amongst caenogastropods (e.g. Vermetidae , Campanilidae ). However, they are unique in the range of midgut morphologies evident (Strong, 2003 and in press) and in the complexity of the female pallial reproductive tract, which bears specialized sperm storage pouches of uncertain homology to those of other caenogastropods.
Although the current concept of Cerithioidea includes 17 Recent families, several additional families that were included in earlier classifications ( Thiele, 1929; Wenz, 1939; Taylor & Sohl, 1962) are now excluded. These are the caenogastropods Campanilidae and Plesiotrochidae (Campaniloidea) , Vermetidae (Vermetoidea) , Caecidae (Rissooidea) , Triphoridae and Cerithiopsidae (Triphoroidea) , Abyssochrysidae (Abyssochrysoidea) , and the lower heterobranchs Architectonicidae (Architectonoidea) and Mathildidae (Mathildoidea). This heterogeneous assemblage was united by features of the shell (mostly tall, conical with numerous whorls, with or without a small siphonal canal) and operculum (corneous, pauci- to multi- spiral) and absence of the male copulatory organ ( Thiele, 1929).
A major restructuring of ‘prosobranch’ relationships brought about in part through new ultrastructural data of the osphradium, led to the removal of several ‘mesogastropods’ to the basal Heterobranchia ( Haszprunar, 1985, 1988), including Valvatidae, Architectonicidae, and Mathildidae. Ultrastructural studies of the eusperm and parasperm have also been highly influential in structuring our current understanding of the composition of the superfamily and have confirmed the affinities of the basal heterobranch taxa ( Healy, 1988 a, 1991, 1993b, 1995). In addition, comparative studies revealed that basal caenogastropods in the Viviparoidea, Cyclophoroidea, Campaniloidea , and Cerithioidea possess similarities in sperm morphology that set them apart from all other caenogastropods, including distinctive features of the eusperm acrosome (conical to flattened, lacking an apical bleb and usually lacking an accessory membrane), eusperm midpiece (often with parallel cristal plates), and parasperm (with head and tail tuft) (see Healy, 1983, 1988a and references therein). Recognition of this common organization supported removal of the Vermetidae , Cerithiopsidae , and Triphoridae ( Healy, 1984, 1988a, 1990; see also Buckland-Nicks & Hadfield, 2005), all of which possess sperm characteristics typical of more derived caenogastropods. Houbrick (1979) removed the Abyssochrysidae based on unique anatomical features including a distinctive radula and pallial penis (later demonstrated to be a pallial tentacle; Ponder & Warén, 1988; Warén & Ponder, 1991) and placed the family in the vicinity of the Zygopleuridae and Pseudozygopleuridae (formerly in the Loxonematoidea, but currently in the ‘zygopleuroid group’; Bouchet & Rocroi, 2005) given the great similarity in shell morphology to these Palaeozoic fossils. Eusperm morphology and the presence of distinctive spermatozeugmata later confirmed that the Abyssochrysidae lies outside the Cerithioidea , but indicated that their affinities lie rather with the Littorinimorpha (Littorinoidea and Rissooidea, in particular; Healy, 1989); new molecular data demonstrate that abyssochrysids are in fact nested within Provannidae ( Johnson et al., 2010) .
Campanilidae View in CoL and Plesiotrochidae View in CoL are the taxa most recently removed from the superfamily. Campanilidae View in CoL contains only one Recent species, Campanile symbolicum Iredale, 1917 View in CoL of Western Australia. Although included in the Cerithioidea by Houbrick (1981a, 1988) and Ponder & Warén (1988), it is now considered to represent a distinct group supported by anatomical data ( Houbrick, 1981 a, 1989) and sperm ( Healy, 1986b, 2000) and osphradial fine structure ( Haszprunar, 1988, 1992). Plesiotrochus View in CoL was formerly classified in the Cerithiidae View in CoL (e.g. Thiele, 1929) and Houbrick (1980) retained the Plesiotrochidae View in CoL within the Cerithioidea when he erected the family. However, eusperm and parasperm of Plesiotrochus View in CoL are similar to those of Campanile View in CoL and the two are currently united in the Campaniloidea ( Healy, 1993a; Healy & Wells, 1998b).
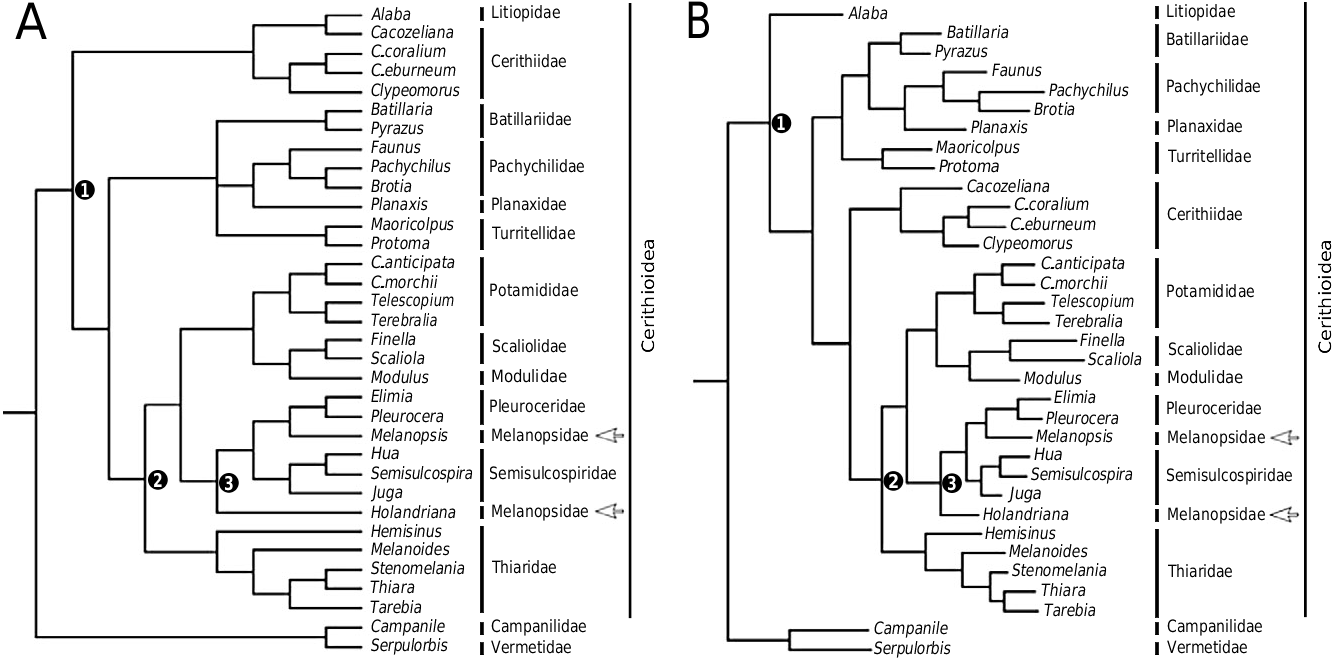
Historically, little attention has been paid to the relationships amongst cerithioidean families and broad family concepts have dominated. For example, the Cerithiidae View in CoL was used to encompass many diverse taxa ranging from Ataxocerithium (Triphoroidea) View in CoL , Campanile View in CoL and Plesiotrochus (Campaniloidea) View in CoL to Diala (Dialidae) View in CoL , and Litiopa (Litiopidae) View in CoL albeit often in several subfamilies. Similarly all limnic cerithioideans were previously included within the Thiaridae View in CoL (often under the invalid name ‘Melaniidae’; e.g. Brot, 1874; Thiele, 1925, 1928, 1929; Sunderbrinck, 1929; Wenz, 1939) despite being acknowledged as a heterogeneous assemblage of unrelated taxa (e.g. Moore, 1897, 1898; Smith, 1904; Pilsbry & Bequaert, 1927). Apart from Thiele (1929), whose classification recognized six distinct freshwater subfamilies that conform broadly to current family-level concepts, the only other attempt to formalize such hypotheses was the classification of Morrison (1954), which supported three freshwater lineages ( Pleuroceridae View in CoL , Melanopsidae View in CoL , Thiaridae View in CoL ) each with independent marine origins. Regrettably, Morrison’s influential classification advanced family concepts that were highly polyphyletic based on the assumed homology of brood structures (see Glaubrecht, 1996). Since that time, the significant new morphological and molecular data produced for freshwater taxa (see above) have largely confirmed Thiele’s view, but these data have not been assessed in a comprehensive cladistic framework and the relationship amongst freshwater lineages is still unclear.
Family-group members of the current concept of Cerithioidea were first formally listed by Ponder & Warén (1988), with the exception of the inclusion of Campanilidae , and most recently by Bouchet & Rocroi (2005). However, phylogenetic analyses of Cerithioidea to date have produced conflicting topologies and have not always supported composition of the ingroup as currently recognized ( Houbrick, 1988; Ponder, 1991; Glaubrecht, 1996; Simone, 2001; Lydeard et al., 2002) (see Figs 2 View Figure 2 , 3 View Figure 3 ). In the first morphology-based phylogenetic analysis of the group, Houbrick (1988) included 14 family-level ingroup terminals, 11 of which are counted amongst the 17 currently recognized, with Rissoa and Strombus as outgroups; 58 morphological characters were coded, including features of the teleoconch, operculum, external anatomy, radula, alimentary, reproductive and nervous systems, and sperm ultrastructure. Houbrick’s view of the classification of cerithioideans at that time was rooted in that of Thiele (1929), with campanilids and vermetids as part of the ingroup, but he acknowledged that some families were likely to be polyphyletic. Houbrick (1988) attempted to mitigate the impact of this, as well as that of high intrafamilial variability, by coding only the nominal taxon for which each family-group name was derived. Consequently, his analysis did not assess monophyly of individual families, but only attempted to establish the relationships amongst them. Houbrick’s results supported Campanilidae as the most basal offshoot, and Pleuroceridae plus Melanopsidae as the third most basal offshoot after Litiopidae . The remaining taxa clustered in two main clades: one with Cerithiidae , Diastomatidae , and Thiaridae and Planaxidae as sister taxa, and a second clade with Vermetidae as sister to Turritellidae , and with Batillariidae , Modulidae , and Potamididae ( Fig. 2A View Figure 2 ). Houbrick (1989) later modified his views and supported placement of Campanilidae in a separate superfamily given a reinterpretation of new and existing characters. The placement of Campanilidae at the base of the tree is consistent with this view, but Vermetidae is placed firmly within the ingroup, which conflicts with current concepts.
Ponder (1991) reanalysed Houbrick’s (1988) data but excluded vermetids and added new morphological information for Diala . Ponder argued that Houbrick’s use of Rissoidae as an outgroup was inadequate to polarize characters given their distant relationship; excluding Rissoidae and using Campanile as the sole outgroup resulted in a reversal of the polarity of the tree in one of the two most parsimonious trees ( Fig. 2B View Figure 2 ) with Turritellidae at the base, demonstrating sensitivity of the results to placement of the root.
Glaubrecht (1996) produced an hypothesis of cerithioidean relationships using Hennigian argumentation involving the weighting and polarizing of 48 anatomical characters by hand (teleoconch, external anatomy, operculum, radula, alimentary and reproductive systems, and sperm ultrastructure) for 13 cerithioidean families; ‘Eucaenogastropoda’, Ampullarioidea, and Cyclophoroidea served as outgroups, and Campanilidae plus Plesiotrochidae were also included ( Fig. 2C View Figure 2 ). In the resulting topology, the Campaniloidea was monophyletic and sister to the Cerithioidea . Within the Cerithioidea , Melanopsidae , Pachychilidae (= Pleuroceridae plus Pachychilidae in part, as currently conceived), Siliquariidae plus Turritellidae and Dialidae plus Litiopidae all form an unresolved polytomy at the base of the tree; the remaining taxa clustered in two clades, one with Cerithiidae , Diastomatidae , Planaxidae plus Thiaridae (= Thiaridae s.s. plus Paludomidae and Pachychilidae in part, as currently conceived), and the other with Batillariidae and Potamididae plus Modulidae .
Simone’s (2001) morphological phylogeny of the superfamily incorporated 19 primarily western Atlantic/neotropical species-level terminals distributed amongst 12 families; Campanilidae and Vermetidae were amongst these to test their exclusion from the superfamily. The morphological data set comprised 122 characters for teleoconch, external anatomy, operculum, renal, alimentary,and reproductive systems, and 22 characters new to cerithioidean systematics relating to buccal musculature; sperm ultrastructure characters were not included. Given Simone’s view of the Cerithioidea as stem-group caenogastropods, a pooled ‘archaeogastropod’ served as the primary outgroup, with the caenogastropod families Viviparidae , Hydrobiidae , and Littorinidae as secondary outgroups. The consensus of three most parsimonious trees placed Modulidae as the most basal offshoot, followed by a clade with Campanilidae as sister to Vermetidae plus Turritellidae ; two freshwater clades were obtained, one with Pachychilidae , and one with Planaxidae as sister to Thiaridae s.s. ( Fig. 2D View Figure 2 ). However, the choice of taxon sampling did not allow rigorous assessment of familial monophyly. Simone also reanalysed Houbrick’s (1988) data using the pooled ‘archeogastropod’ as the outgroup, and similarly recovered a tree with essentially reversed polarity, placing Turritellidae plus Vermetidae at the base and Campanilidae as the second offshoot, recapitulating Ponder’s (1991) findings with regards to rooting sensitivity.
In the only molecular phylogenetic analysis of the superfamily, Lydeard et al. (2002) analysed DNA sequences from mitochondrial large subunit rRNA and flanking tRNA genes. The data set comprised 40 nearly full-length sequences (32 cerithioideans, eight outgroups) with a total aligned length of 1873 bp. This analysis is the most comprehensive to date, allowing evaluation of monophyly of a number of cerithioidean families for the first time. Parsimony analysis resulted in four equally parsimonious trees with the strict consensus tree shown in Figure 3A; a View Figure 3 slightly different topology was recovered with transversion weighting ( Fig. 3B View Figure 3 ). Monophyly of the Cerithioidea as currently conceived was supported with Campanile plus Serpulorbis as the sister group to the Cerithioidea . The exclusion of Campanilidae and Vermetidae was supported by a unique tRNA gene order arrangement, with mitochondrial small subunit (mtSSU)-thr-gly-val- mitochondrial large subunit (mtLSU) in all cerithioideans versus mtSSU-val-mtLSU in other caenogastropods. Amongst families with more than a single representative in the analysis, both equally weighted and differentially weighted topologies supported monophyly of the Batillariidae , Pachychilidae , Pleuroceridae , Potamididae , Scaliolidae , Semisulcospiridae , Thiaridae , and Turritellidae . Cerithiidae was monophyletic in the differentially weighted but not the equally weighted analysis, whereas Melanopsidae was polyphyletic in both analyses.
In summary, existing morphological phylogenies have had limited capacity to assess familial monophyly and do not reflect significant new data generated in recent years (see above). In addition, there is considerable disagreement amongst the analyses produced thus far and no analysis has explored simultaneous analysis of morphological and molecular information. The goal of the present analysis is to generate an updated morphological data set for an expanded selection of taxa in the most inclusive morphological analysis of the superfamily to date. The molecular data of Lydeard et al. (2002), pertinent sequences that have become available since, and newly generated 28S sequences are synthesized and analysed separately and together with the morphological data, to provide the best estimate of phylogenetic relationships within the group possible with current information.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pachychilus sp.
| Strong, Ellen E., Colgan, Donald J., Healy, John M., Lydeard, Charles, Ponder, Winston F. & Glaubrecht, Matthias 2011 |
Campanile symbolicum
| Iredale 1917 |
Campanilidae
| Douville 1904 |
Campanilidae
| Douville 1904 |
Campaniloidea
| Douville 1904 |
Pleuroceridae
| P. Fischer 1885 |
Campanile
| P. Fischer 1884 |
Campanile
| P. Fischer 1884 |
Thiaridae
| Gill 1871 |
Thiaridae
| Gill 1871 |
Diala (Dialidae)
| A. Adams 1861 |
Melanopsidae
| H. Adams & A. Adams 1854 |
Litiopa (Litiopidae)
| Rang 1829 |
Cerithioidea
| J. Fleming 1822 |
Cerithiidae
| J. Fleming 1822 |
Cerithioidea
| J. Fleming 1822 |
Cerithiidae
| J. Fleming 1822 |