Leptodactylus kilombo, Da Silva & Magalhães & Thomassen & Leite & Garda & Brandão & Haddad & Giaretta & De Carvalho, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4779.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:C5769EAA-CAF6-441F-A22F-D483E7E969C7 |
|
DOI |
https://doi.org/10.5281/zenodo.3851229 |
|
persistent identifier |
https://treatment.plazi.org/id/E9291B2E-D63E-49AE-9CDD-122560774016 |
|
taxon LSID |
lsid:zoobank.org:act:E9291B2E-D63E-49AE-9CDD-122560774016 |
|
treatment provided by |
Plazi |
|
scientific name |
Leptodactylus kilombo |
| status |
sp. nov. |
Leptodactylus kilombo sp. nov.
( Figs. 2 View FIGURE 2 , 5d View FIGURE 5 , 6f View FIGURE 6 , 8 View FIGURE 8 )
[http://zoobank.org/ urn:lsid:zoobank.org:act:E9291B2E-D63E-49AE-9CDD-122560774016 ]
Leptodactylus sp. ( aff. mystaceus ): Silva et al. (2020)
Holotype. CHUFPB 28204 , an adult male from Bezerra stream (12.897899°S, 46.810419°W; 626 m above sea level [a.s.l.]), in Arraias, Tocantins State, northern Brazil, collected by L. A. da Silva on 7 November 2018. GoogleMaps
Paratypes. One adult male ( CHUFPB 28205 ) and one adult female ( CHUFPB 30409 ) collected with the holotype .
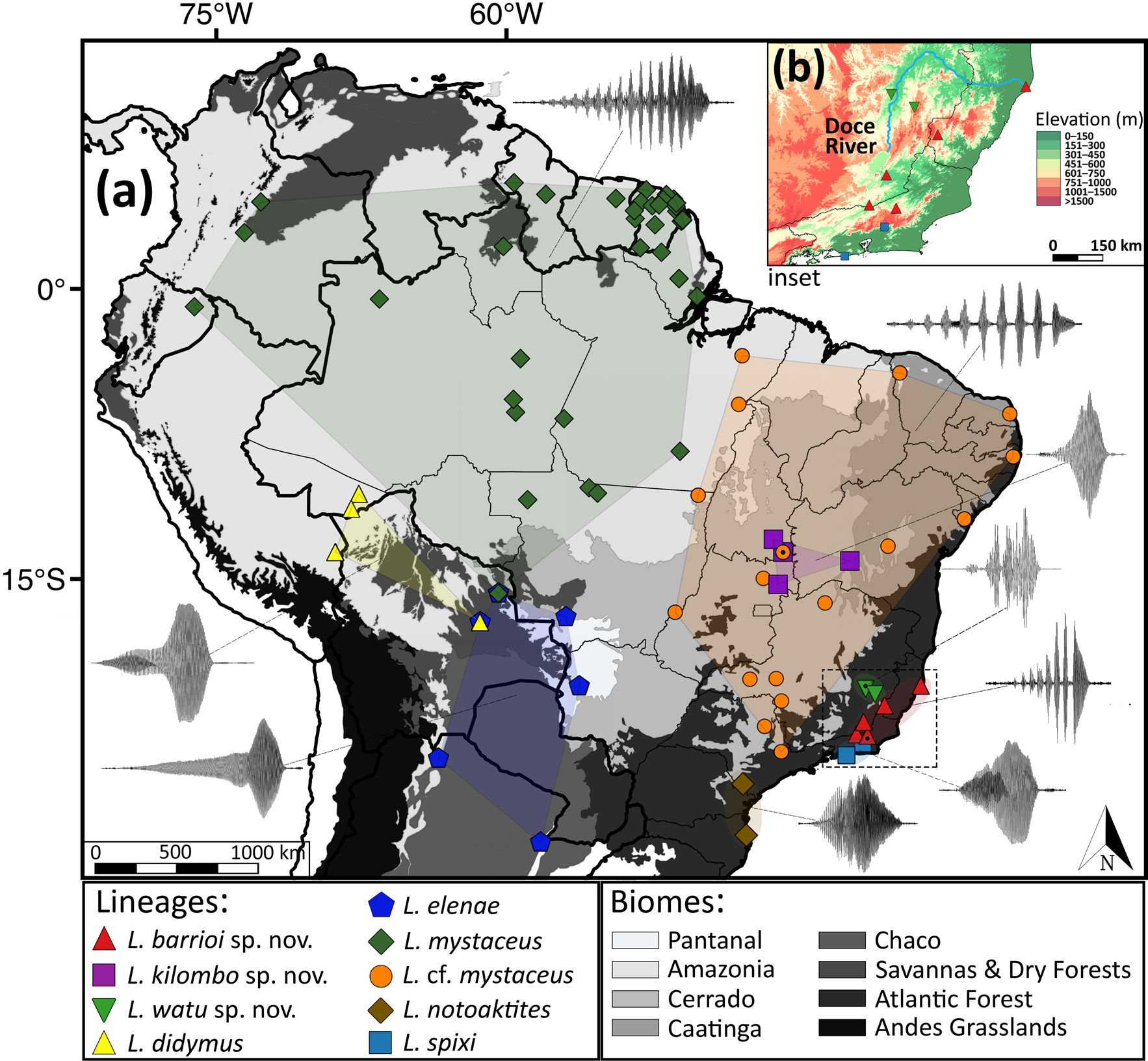
Referred material. One genetic voucher ( UFBA 7694) from Bom Jesus da Lapa, Bahia State ( Fig. 1 View FIGURE 1 ), originally assigned to L. mystacinus by Dória et al. (2018).
Etymology. The Kimbundu word kilombo (quilombo in Portuguese), used as a noun in apposition, literally means war camp. Quilombos are hinterland settlements founded by people of African origin including the quilombolas (inhabitants of quilombos), maroons, and some other names and variations by which these communities recognize themselves. Most of the original inhabitants were runaway slaves. There are hundreds of recognized quilombos throughout Brazil and also several out of record by the federal government. Such settlements carry plenty of historical, cultural, and anthropological history back into the Brazilian Imperial Age (colonial Brazil), especially in the mid-17th century, related to the slavery in the country. The type locality of Leptodactylus kilombo is located nearby an ancient quilombola settlement named Chapada dos Negros in Arraias, in the south of Tocantins State, north central Brazil ( Gualberto 2017).
Diagnosis. Leptodactylus kilombo is characterized by the following combination of character states: (1) small to moderate size, male SVL = 37.6–41.3 mm, female SVL = 41.8 mm; (2) advertisement call composed of single, nonpulsed notes; (3) note duration varying from 30–56 ms; (4) note dominant frequency varying from 1098–1572 Hz; (5) frequency upsweep varying from 388–775 Hz.
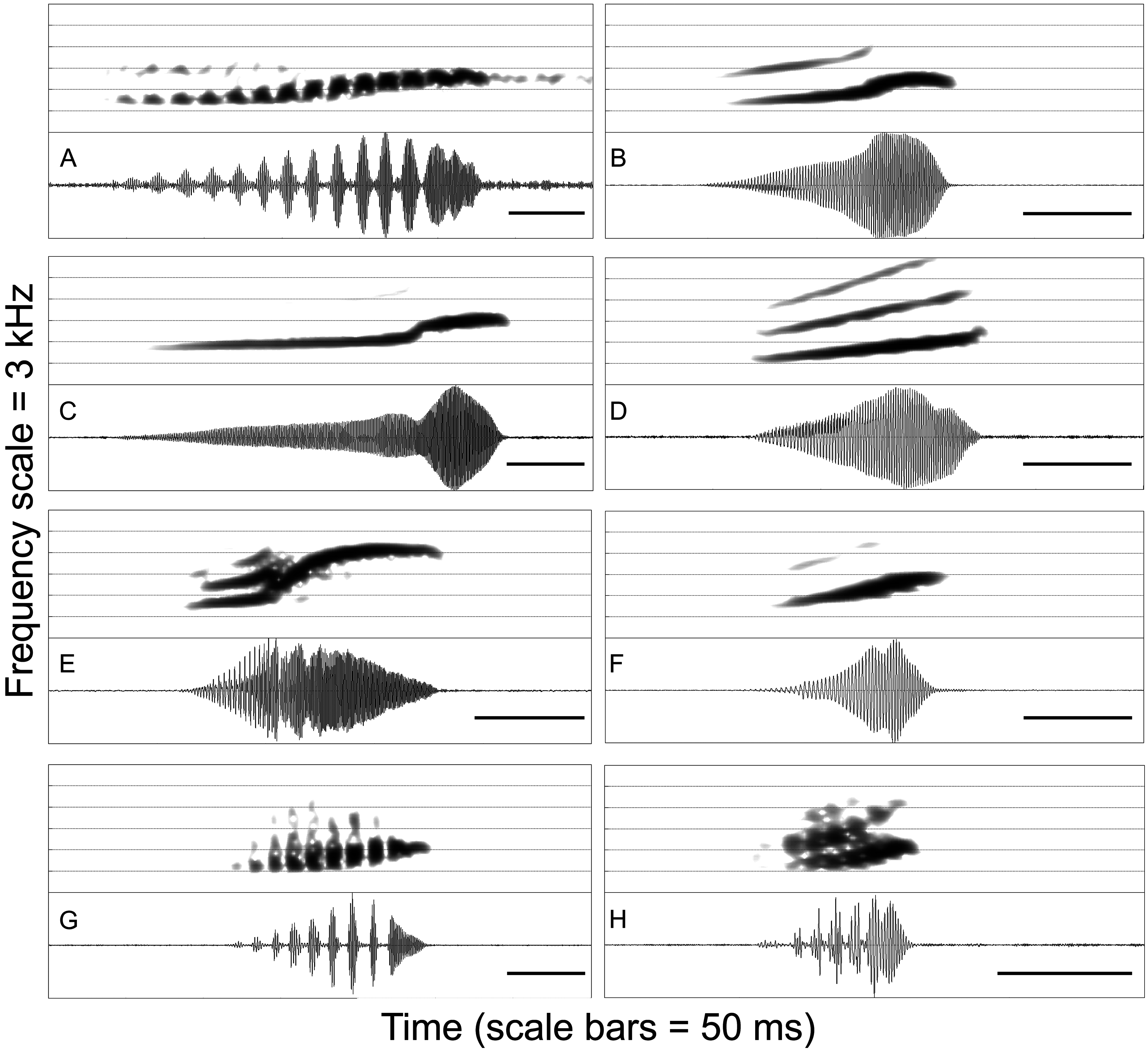
Comparisons with members of the L. mystaceus complex. Leptodactylus kilombo can be distinguished from other species by having the sole of the foot covered with few, non-obvious tubercles (prominent tubercles in the other species; Heyer 1978, 1983; Heyer et al. 1996), except Leptodactylus notoaktites . The new species (male SVL = 37.6–41.3 mm, mean = 40.8; female SVL = 41.8 mm; Table 2) can be further distinguished from the other species of the L. mystaceus complex, except L. elenae and L. spixi , by a smaller size: L. didymus (male SVL = 45.9–52.2 mm, mean = 49.3; female SVL = 45.8–53.5 mm, mean = 49.8; Heyer et al. 1996), L. mystaceus (male SVL = 42.4–52.2 mm, mean = 47.4; female SVL = 44.8–56.1 mm, mean = 50.0; Heyer et al. 1996), L. notoaktites (male SVL = 42.5–54.2 mm, mean = 47.5; female SVL = 43.4–55.8 mm, mean = 48.0; de Sá et al. 2014). Members of the L. mystaceus complex are mainly distinguished from each other by their distinct advertisement calls ( Fig. 6 View FIGURE 6 ).
Acoustic comparisons in the L. fuscus group. The advertisement call of Leptodactylus kilombo is composed of single notes ( Fig. 6f View FIGURE 6 ), differing from the trill calls of L. cunicularius , L. cupreus , L. oreomantis , and L. plaumanni ( Fig. 7 View FIGURE 7 ; Carvalho et al. 2013). The new species is distinguished from species with pulsed calls ( L. caatingae , L. fragilis , L. labrosus , L. mystaceus , and L. cf. mystaceus ; Heyer 1978; Heyer & Juncá 2003; Heyer et al. 2006; Carvalho & Ron 2011) by having a nonpulsed call. From the remaining species of the L. fuscus group also having single-note, nonpulsed calls, L. kilombo is distinguished by a shorter note duration (30–56 ms) from L. bufonius , L. camaquara , L. didymus , L. elenae , L. laticeps , and L. spixi (> 100 ms; Table 3; Heyer & Scott 2006; Carvalho et al. 2013). The call of L. kilombo has a modest frequency upsweep (frequency modulation, FM = 388–775 Hz), whereas L. albilabris , L. fuscus , L. gracilis , L. jolyi , L. longirostris , L. notoaktites , L. sertanejo , and L. syphax have a pronounced frequency upsweep in their calls (FM> 1000 Hz; Heyer 1978; Giaretta & Costa 2007; Heyer et al. 2010), while that of L. poecilochilus has a negligible/slight frequency upsweep (FM <200 Hz; Heyer 1978). Leptodactylus kilombo is distinguished by a lower dominant frequency (1098–1572 Hz) from L. furnarius , L. latinasus , L. marambaiae , L. mystacinus , L. tapiti , and L. troglodytes (> 2000 Hz) ( Heyer 1978; Sazima & Bokermann 1978; Brandão et al. 2013).
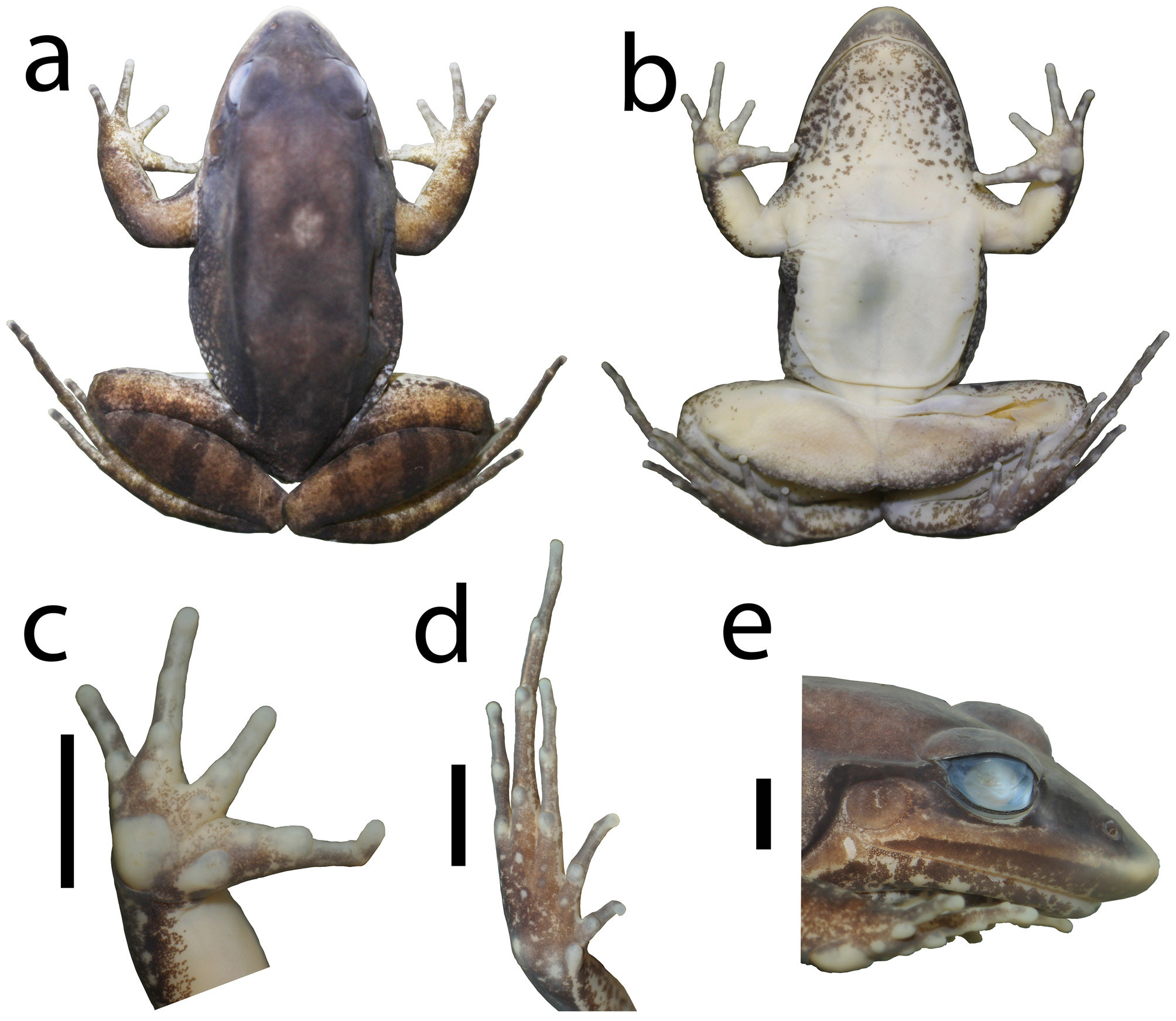
Description of holotype. Body robust. Snout sub-elliptical in dorsal and ventral views ( Fig. 8 View FIGURE 8 a–b), acuminate in lateral view ( Fig. 8e View FIGURE 8 ). Canthus rostralis rounded; loreal region flat; tympanic annulus well-defined, circular (TD = 60.4% ED); tympanic membrane translucent (brownish); supratympanic fold from the posterior corner of the eye, passing over the dorsal edge of the tympanic annulus and ending at the base of the arm; vocal sac subgular; vocal slits present; vomerine teeth in two nearly straight rows medial and posterior to choanae and almost parallel to sagittal plane. Tongue rounded, free at its posterior third. Relative finger lengths IV <II <I <III; fingers without lateral fringing or webbing; finger tips rounded, unexpanded; inner metacarpal tubercle oblong, single; outer metacarpal tubercle triangular; outer metacarpal tubercle twice the width of the inner ( Fig. 8c View FIGURE 8 ). Subarticular tubercles rounded; supernumerary tubercles absent. Dorsal surfaces of body and forelimbs smooth, shagreened on flank and dorsal surface of shank. Dorsolateral fold from the posterior corner of the eye, extending posteriorly to the groin. A lateral line of tubercles on flank restricted to posterior flank. Dorsal surface of hindlimbs covered with tubercles (more conspicuous in life); posterior surface of tarsus covered with tiny tubercles. Ventral surface of body and limbs smooth, underside of thigh areolate. Relative toe lengths I <II <V <III <IV; toes without lateral fringing or webbing; toe tips unexpanded ( Fig. 8d View FIGURE 8 ). Inner metatarsal tubercle elongated, twice the maximum length of the nearly rounded outer metatarsal tubercle. Tarsal fold extending 2/3 of tarsus length, from the inner metatarsal tubercle towards the heel. Subarticular tubercles rounded; sole of foot with few tiny tubercles, inconspicuous.
Colors. In life, dorsal surfaces (body and limbs) reddish; snout whitish. Light brown blotches scattered on the dorsum ( Fig. 5d View FIGURE 5 ). Upper portion of the loreal region dark brown with green specks, lower portion reddish on a cream-colored background, lips nonpigmented with light-colored spots and stains. Flank light gray; tubercles reddish and light cream. Throat white scattered with dark brown spots and dots. Chest and belly off-white; ventral surface of limbs cream-colored. Upper surface of thigh, tibia and tarsus brown with dark brown transversal bars. Posterior surface of thigh with brown spots and irregular longitudinal stripe light cream. In preservative, dorsal surfaces (body and limbs) brown with darker brown blotches ( Fig. 8 View FIGURE 8 ). The dorsal reddish coloration in life becomes brown or dark brown. Dark brown blotches and brown transversal bars on the upper surface of thigh and tibia are more conspicuous. Interorbital blotch poorly delimited posteriorly. Upper portion of the loreal region blackish brown, lower portion light brown, lips dark brown. Tympanic membrane light brown. Flank light gray. Throat white covered with dark brown spots and dots. Chest, belly, and ventral surface of limbs varying from cream-colored to off-white. Dark brown transversal bars on upper surface of thigh, tibia and tarsus. Posterior surface of thigh with scattered brown spots and irregular longitudinal stripe stripe light cream. Ventral surface of hand and foot brown interspersed with nonpigmented areas; Sole of foot with sparse white dots.
Intraspecific variation. Lateral line of tubercles is complete in CHUFPB 28205. Posterior surface of tarsus can be smooth or covered with tiny tubercles (poorly visible in preservative). Tubercles on the sole of foot are more conspicuous in the examined female (CHUFPB 30409) than in male specimens.
Advertisement call. Description based on 94 calls recorded from two males ( Table 3). The call ( Fig. 6f View FIGURE 6 ) consists of single, nonpulsed notes given at a rate of 109–122 per minute. Note duration varies from 30–56 ms. Rise time is 37–77% of note duration. The dominant frequency coincides with the fundamental harmonic at 1098–1572 Hz. Notes usually have a modest frequency upsweep throughout their duration, varying from 388–775 Hz.
plex. Data are presented as mean SD (range).
Habitat and natural history. At the type locality, we first found the species in breeding activity early in the rainy season (October 2018), when many calling males could be heard along a dried-up stream (Bezerra stream), which was humid in the sandy stream bed. On this occasion, most males were calling from underneath dense layer of leaf litter (see a calling male at: https://www.youtube.com/watch?v=SUyRQOiGReQ). During a second expedition to the type locality later in the rainy season (April 2019), we did not find or hear any specimen of the new species, suggesting that its breeding activity may be restricted to the early rainy season. Despite being located in a central region of the Cerrado domain, the type locality is more characterized by the presence of Seasonally Dry Tropical Forests ( Werneck 2011) than typical savanna formations. Leptodactylus kilombo and L. cf. mystaceus are syntopic at the type locality ( Fig. 1 View FIGURE 1 ). Despite being quite similar to each other in overall morphology, these species can be easily distinguished by their advertisement calls (see “Comparisons with congeners” section). Besides L. cf. mystaceus , two other species of the L. fuscus group occur syntopically at the type locality ( L. fuscus and L. troglodytes ). All four species were observed calling simultaneously at the type locality.
Distribution. Leptodactylus kilombo ( Fig. 1 View FIGURE 1 ) is known from two localities in the extreme southeast of Tocantins, the type locality (Arraias) and Conceição do Tocantins, from a single locality in northeastern Goiás (Flores de Goiás; see Appendix II) and a single locality in western Bahia (Bom Jesus da Lapa; see Appendix II).
Remarks. Live specimens of Leptodactylus kilombo have a reddish-pink upper lip stripe. This color feature might be an additional diagnostic feature of the species in the L. mystaceus complex. Because of our small sample size of L. kilombo , further sampling is needed to confirm whether upper lip stripe coloration is an informative character in the diagnosis of this species. We examined three specimens collected from Conceição do Tocantins (field# RAB 1965–67), located approximately 85 km northwest of the type locality of L. kilombo . These specimens resemble L. kilombo in overall morphology and by having a reddish-pink upper lip stripe, thus assigned to L. kilombo based on morphological and color features from the preserved specimens and pictures in life, but acoustic and molecular data should also be obtained from this population.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |