Leptodactylus watu, Da Silva & Magalhães & Thomassen & Leite & Garda & Brandão & Haddad & Giaretta & De Carvalho, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4779.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:C5769EAA-CAF6-441F-A22F-D483E7E969C7 |
|
DOI |
https://doi.org/10.5281/zenodo.3851227 |
|
persistent identifier |
https://treatment.plazi.org/id/5D79A081-C50C-463A-8101-04351E87AD23 |
|
taxon LSID |
lsid:zoobank.org:act:5D79A081-C50C-463A-8101-04351E87AD23 |
|
treatment provided by |
Plazi |
|
scientific name |
Leptodactylus watu |
| status |
sp. nov. |
Leptodactylus watu sp. nov.
( Figs. 2 View FIGURE 2 , 4a, 4c View FIGURE 4 , 5h View FIGURE 5 , 6h View FIGURE 6 , 10 View FIGURE 10 ) [http://zoobank.org/ urn:lsid:zoobank.org:act:5D79A081-C50C-463A-8101-04351E87AD23 ]
Leptodactylus aff. spixi: Guimarães et al. (2019) View in CoL
Holotype. UFMG 21332 View Materials , an adult male from Parque Estadual do Rio Doce (19.753922°S, 42.633047°W, approximately 280 m a.s.l.), in Marliéria, Minas Gerais State, southeastern Brazil, collected by H. Thomassen on 21 November 2017. GoogleMaps
Paratypes. Seven adult males ( UFMG 21327–21331 View Materials and 21337–21338) and five adult females ( UFMG 21333– 21336 View Materials and 21339) collected from the type locality by H. Thomassen from November 2017 to February 2018.
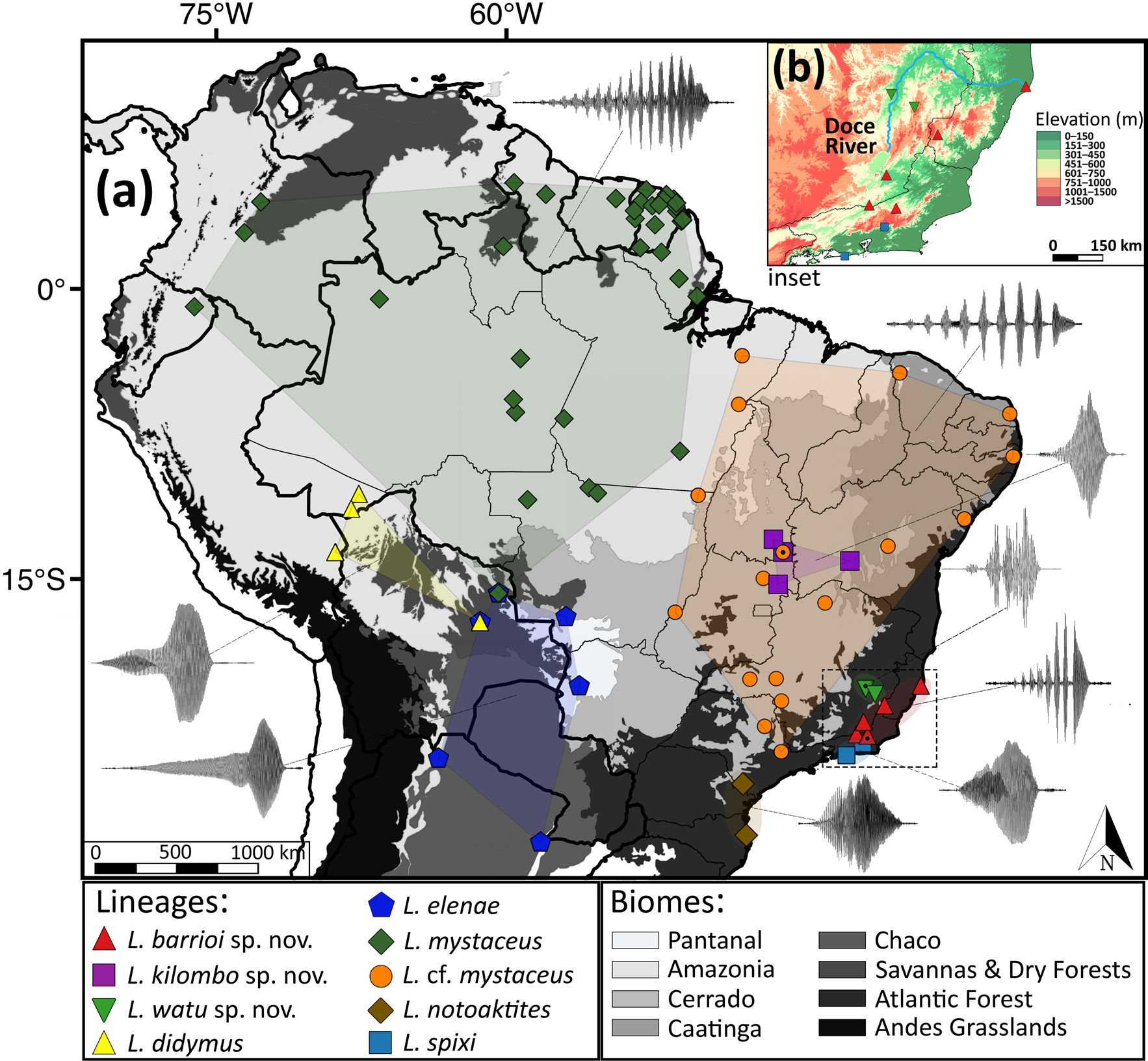
Additional material. One genetic voucher ( UFMG 16995) from Santa Bárbara do Leste, Minas Gerais State ( Fig. 1 View FIGURE 1 ).
Etymology. The word watu , a noun in apposition, is derived from the dialect of the Borún indigenous people and is a reference to the Doce River ( Costa-Reis & Genovez 2013). In 2015, the Doce River experienced the worst environmental disaster that ever took place in Brazil, the collapse of a mining tailings dam owned by Samarco (and co-owned by the Brazilian Vale and Australian BHP Billiton). The catastrophic dam failure released around 60 million cubic meters of iron ore tailings (toxic slurry) directly into the Doce River watershed, killing 20 people and affecting biodiversity across hundreds of kilometers around the river drainage, riparian lands, and the Atlantic coast, in addition to a severe contamination of the soil and water table within the affected region. The epithet is a tribute to the resistance of the Borún people and to the Watu (Doce River) in southeastern Brazil.
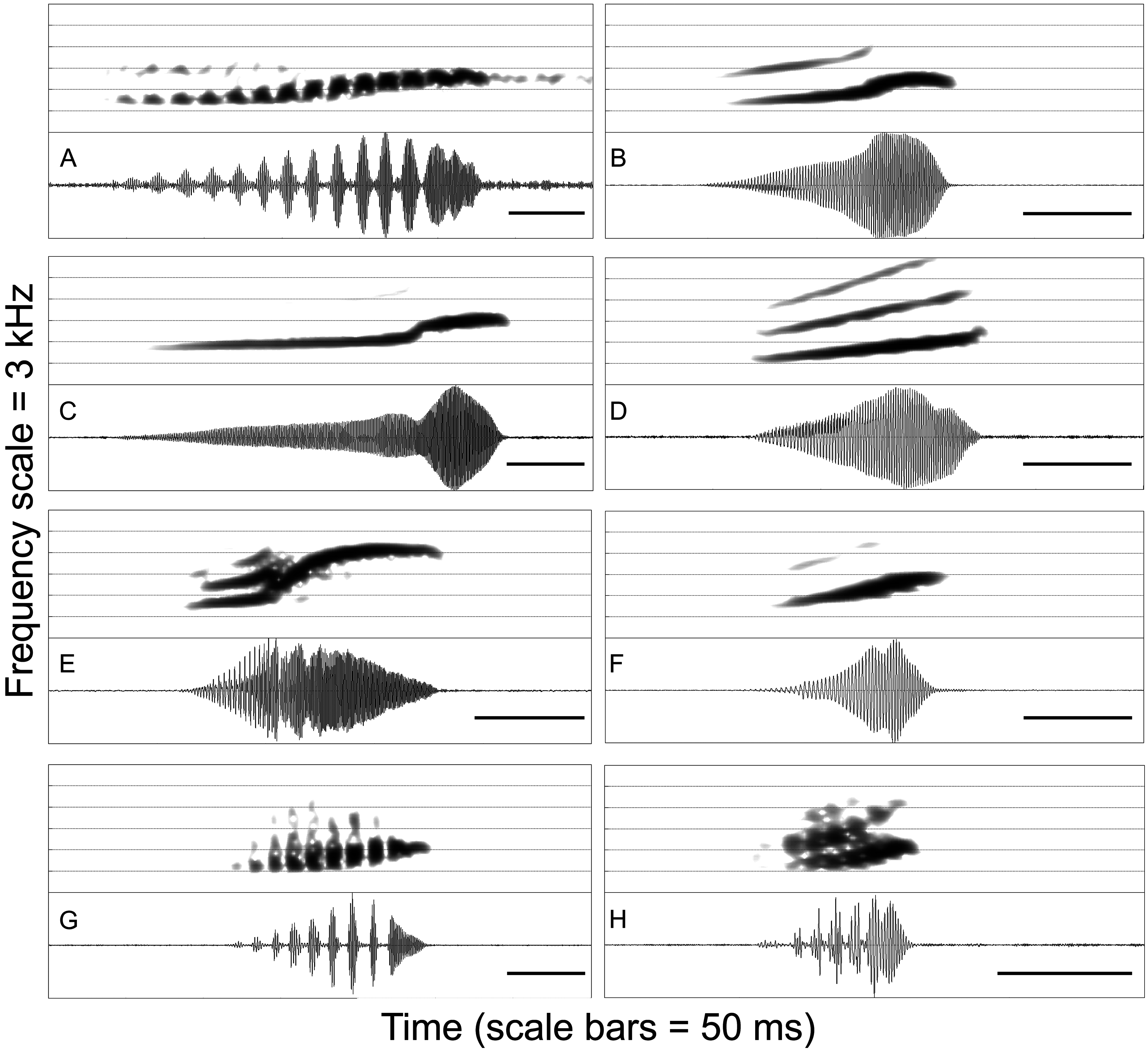
Diagnosis. Leptodactylus watu is characterized by the following combination of character states: (1) small to moderate size, male SVL = 38.5–42.2 mm, female SVL = 40.5–47.9 mm; (2) sole of foot with few, non-obvious tubercles; (3) advertisement call composed of single, pulsed notes; (4) note duration varying from 27–70 ms; (5) note dominant frequency varying from 495–1098 Hz; (6) frequency upsweep varying from 0–560 Hz.
Comparisons with members of the L. mystaceus complex. Leptodactylus watu is distinguished from other species belonging to the L. mystaceus complex, except L. barrioi , L. kilombo and L. notoaktites , by having the sole of the foot covered with few, non-obvious tubercles, whereas L. didymus , L. elenae , L. mystaceus , and L. spixi have prominent tubercles ( Heyer 1978, 1983; Heyer et al. 1996). The new species can be further distinguished from some species by a smaller size ( Table 2): L. didymus (male SVL = 45.9–52.2 mm, mean = 49.3; female SVL = 45.8–53.5 mm, mean = 49.8; Heyer et al. 1996), L. mystaceus (male SVL = 42.4–52.2 mm, mean = 47.4; female SVL = 44.8– 56.1 mm, mean = 50.0; Heyer et al. 1996), and L. notoaktites (male SVL = 42.5–54.2 mm, mean = 47.5; female SVL = 43.4–55.8 mm, mean = 48.0; de Sá et al. 2014). Members of the L. mystaceus complex are mainly distinguished from each other by their distinct advertisement calls ( Fig. 6 View FIGURE 6 ).
Acoustic comparisons in the L. fuscus group. The advertisement call of Leptodactylus watu is composed of single, pulsed notes ( Table 3; Fig. 6h View FIGURE 6 ), differing from species with trill calls ( L. cunicularius , L. cupreus , L. oreomantis , and L. plaumanni ; Fig. 7 View FIGURE 7 ; Carvalho et al. 2013) and species with nonpulsed calls ( L. albilabris , L. bufonius , L. camaquara , L. didymus , L. elenae , L. furnarius , L. fuscus , L. gracilis , L. jolyi , L. kilombo sp. nov., L. laticeps , L. latinasus , L. longirostris , L. marambaiae , L. mystacinus , L. notoaktites , L. poecilochilus , L. sertanejo , L. spixi , L. syphax , L. tapiti , and L. troglodytes ). From other species with pulsed calls, L. watu is distinguished by having its call formed by partly fused pulses, whereas the calls of L. barrioi , L. mystaceus and L. cf. mystaceus are formed by complete pulses ( Fig. 6 View FIGURE 6 ). From species also having calls with partly fused pulses ( L. caatingae , L. fragilis , and L. labrosus ), L. watu is distinguished by a shorter note (27–70 ms) from L. fragilis (> 100 ms; Heyer et al. 2006). The calls of L. watu , L. caatingae , and L. labrosus are the most similar to each other in the L. fuscus group, but these species can be distinguished, although value ranges partially overlap, by differences in the dominant frequency ( L. watu : 495–1098 Hz, mean = 763; L. caatingae : 1148–1430 Hz, mean = 1315, in our sample; 943–1620 Hz, mean = 1423 Hz, in the original description; Heyer & Juncá 2003) or duration ( L. watu : 27–70 ms, mean = 40; L. labrosus : 64–133 ms, mean = 100; Carvalho & Ron 2011) of their calls. Still, these species differ markedly in morphology and size (see previous paragraph; Heyer 1978; Heyer & Juncá 2003).
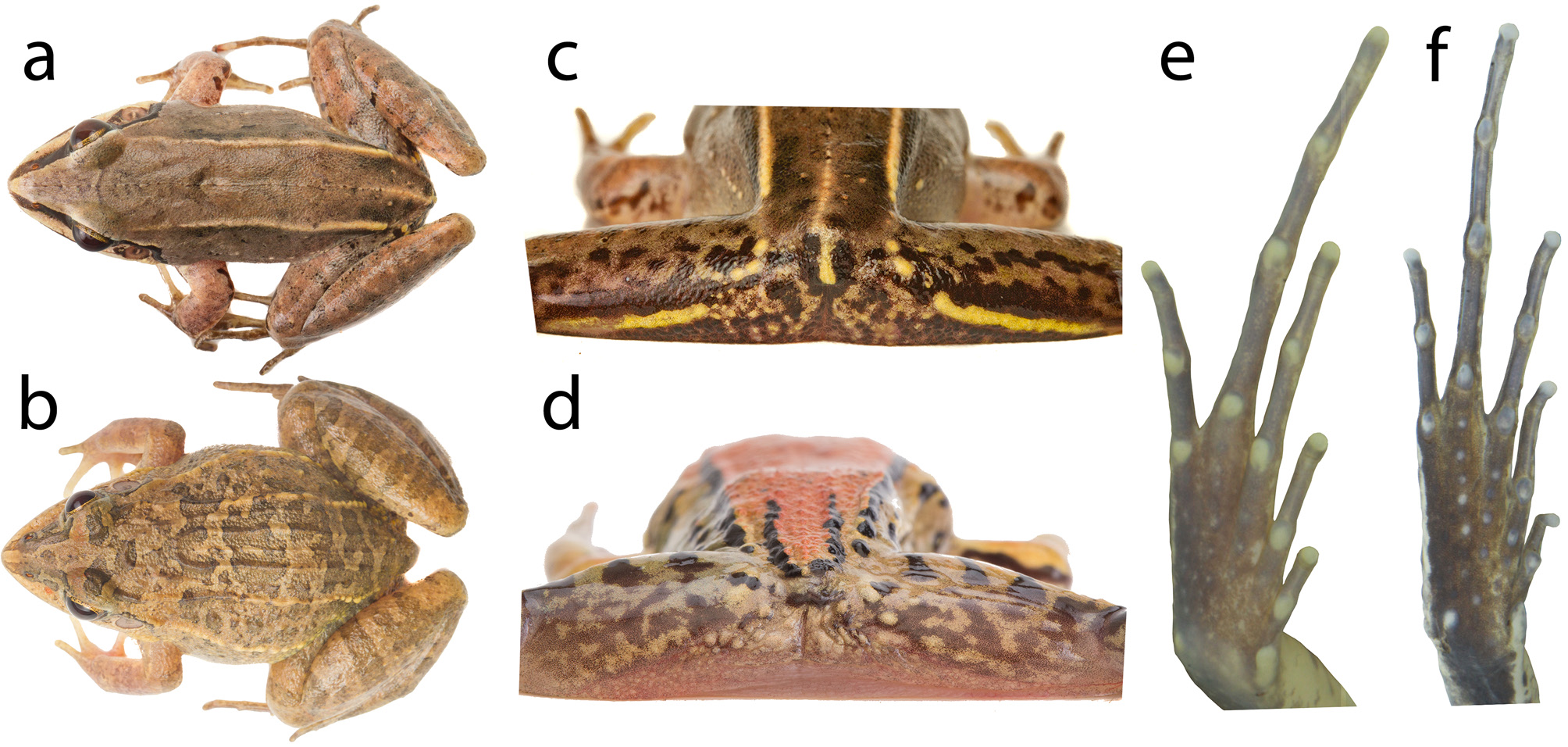
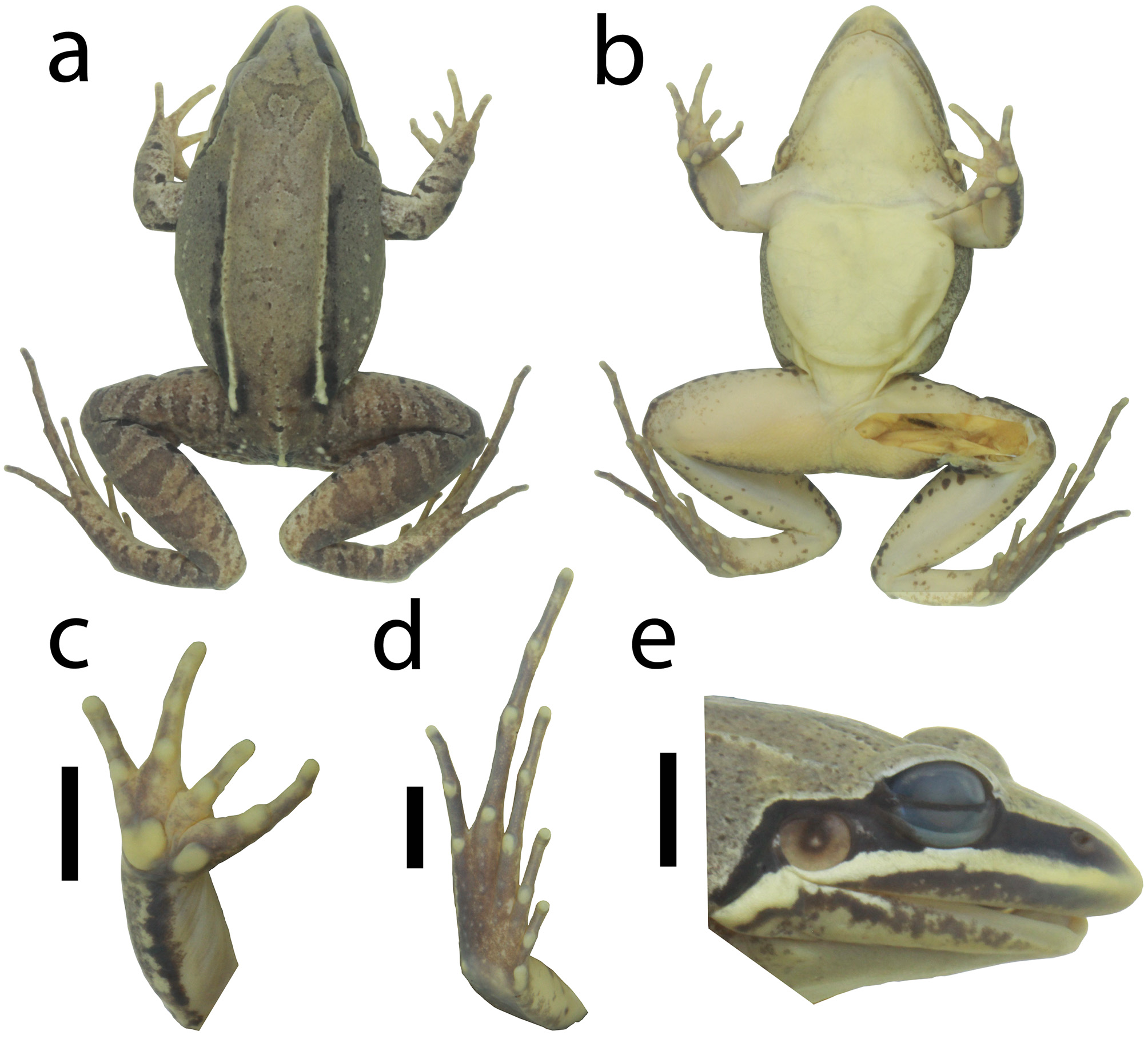
Description of holotype. Body robust. Snout sub-elliptical in dorsal and ventral views ( Fig. 10 View FIGURE 10 a–b), acuminate in lateral view ( Fig. 10e View FIGURE 10 ). Canthus rostralis rounded; loreal region flat; tympanic annulus well-defined, circular (TD = 62.4% ED); supratympanic fold from the posterior corner of the eye, passing over the dorsal edge of the tympanic annulus, and ending at the base of the arm; vocal sac subgular; vocal slits present; vomerine teeth in two nearly straight rows, almost connected, medial and posterior to choanae and almost parallel to sagittal plane. Tongue rounded, free at its posterior third. Relative finger lengths II = IV <I <III; fingers without lateral fringing or webbing; finger tips rounded, unexpanded; inner metacarpal tubercle oval, divided, outer metacarpal tubercle rounded; outer metacarpal tubercle twice the maximum width of the inner; subarticular tubercles rounded; supernumerary tubercles absent ( Fig. 9c View FIGURE 9 ). Dorsal surfaces of body and forelimbs smooth, shagreened on flank and dorsal surface of shank. Dorsolateral fold from the posterior corner of the eye, extending posteriorly to the groin. Flank with a fragmented line of granules posteriorly. Dorsal surface of hindlimbs with tiny tubercles (more conspicuous in life). Ventral surface of body and limbs smooth, underside of thigh areolate. Relative toe lengths I <II <V <III <IV; toes without lateral fringing or webbing; toe tips unexpanded. Inner metatarsal tubercle elongated, twice the maximum length of the ovoid outer metatarsal tubercle ( Fig. 10d View FIGURE 10 ). Tarsal fold extending 2/3 of tarsus length, from the inner metatarsal tubercle towards the heel; subarticular tubercles rounded; sole of foot with tiny tubercles, inconspicuous.
Colors. In life, dorsal surfaces (body and limbs) light brown with dark gray blotches poorly delimited ( Figs. 4a, c View FIGURE 4 , 5h View FIGURE 5 ). Upper region of the loreal region black, lower region light cream, lips dark brown. Black stripe on the supratympanic fold, delimited dorsally by a white line. Light cream stripe on the dorsolateral fold, more conspicuous posteriorly. Flank light gray. Tympanic membrane dark brown on the center and upper rim, light brown laterally and on ventral rim. Throat immaculate white with nonpigmented portions near the jaw. Ventral surface of arms, legs (except tarsus), and belly nonpigmented; tarsus with scattered dark brown stains. Weakly defined dark brown transversal bars on the upper surface of hindlimbs and forearm. Posterior surface of thigh with a yellow longitudinal stripe bordered by two black stains; upper portion of with black spots and stains. Ventral surface of hand, foot, and tarsus dark brown interspersed with nonpigmented areas. In preservative, dorsal surfaces (body and hindlimbs) light gray with dark gray blotches poorly delimited; forearms light cream with scattered dark gray dots and spots ( Fig. 10 View FIGURE 10 ). Upper portion of the loreal region black, lower half cream-colored, lips blackish gray. Black stripe on the supratympanic fold. White stripe on the dorsolateral fold, more conspicuous posteriorly. Flank light gray. Tympanic membrane light brown with the center and upper edge dark gray. Ventral surfaces cream-colored. Throat with few gray dots and stains near the jaw laterally. Ventral surface of hindlimbs with few dark gray spots. Dark brown transversal bars on the dorsal surface of hindlimbs and forearm. Posterior surface of thigh with a white longitudinal stripe bordered by two black stains. Ventral surface of hand, foot, and tarsus dark brown interspersed with nonpigmented areas.
Intraspecific variation. In life, dorsal coloration varies from brown, sometimes conspicuous reddish shades, or dark gray. The dorsal light brown coloration in life becomes light gray in preservative. Dark brown blotches on the dorsum can be present, more conspicuous in preserved specimens. Dark brown transversal bars on the upper surface of thigh and tibia are more evident in preserved species. Lateral line of tubercles is poorly evident in most specimens. Inner metacarpal tubercle is rounded in UFMG 21329.
Advertisement call. Description based on 129 calls recorded from two males ( Table 3). The call ( Fig. 6h View FIGURE 6 ) consists of single, pulsed notes given at a rate of 124–275 per minute. Note duration varies from 27–70 ms. Rise time varies from 37–80% of note duration. Notes are formed by 2–7 partly fused pulses emitted at a rate of 57–153 per second. The dominant frequency coincides with the fundamental harmonic at 495–1098 Hz. Notes have no frequency modulation or a modest frequency upsweep throughout their duration, varying from 0–560 Hz.
Habitat and natural history. At the type locality, Leptodactylus watu was found calling during the rainy season in the region from late October to late February. Calling is strongly associated with rainy days or few days after heavy pours. Males were found calling around flooded areas or close to moist ground in both open areas (transitional marshlands between the forest and lakes) and inside the forest. All calling males were hidden either under dense vegetation, ground leaf litter or inside underground chambers. They were never found calling in pastures. The congeneric species found calling in syntopy with L. watu were L. fuscus , L. latrans , and L. labyrinthicus .
Distribution. Leptodactylus watu is only known from two localities in a region characterized by lowland interior Atlantic forests associated with the upper-middle Doce River drainage, in the Brazilian state of Minas Gerais ( Fig. 1 View FIGURE 1 ): (1) the type locality, at Parque Estadual do Rio Doce, and (2) Santa Bárbara do Leste, approximately 70 km southeast from the type locality.
Remarks. The identity of populations assigned to Leptodactylus aff. mystaceus ( Santana et al. 2010) , L. aff. spixi ( Ferreira et al. 2019) , and L. spixi ( Rödder et al. 2007; Silva-Soares & Scherrer 2013; Pereira-Ribeiro et al. 2019) from different localities in southeastern and northeastern Brazil should be addressed using calls and/or DNA sequences, as these records could correspond to L. barrioi or L. watu , or additional candidate new species.
| UFMG |
Universidade Federal de Minas Gerais |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Leptodactylus watu
| Da Silva, Leandro A., Magalhães, Felipe M., Thomassen, Hans, Leite, Felipe S. F., Garda, Adrian A., Brandão, Reuber A., Haddad, Célio F. B., Giaretta, Ariovaldo A. & De Carvalho, Thiago R. 2020 |
Leptodactylus aff. spixi: Guimarães et al. (2019)
| Guimaraes 2019 |