Oxypoda brachyptera ( STEPHENS , 1832)
|
publication ID |
https://doi.org/ 10.21248/contrib.entomol.62.1.207-224 |
|
DOI |
https://doi.org/10.5281/zenodo.4812783 |
|
persistent identifier |
https://treatment.plazi.org/id/4E368798-9D30-FFE4-2381-FF53FE47FAEF |
|
treatment provided by |
Carolina |
|
scientific name |
Oxypoda brachyptera ( STEPHENS , 1832) |
| status |
|
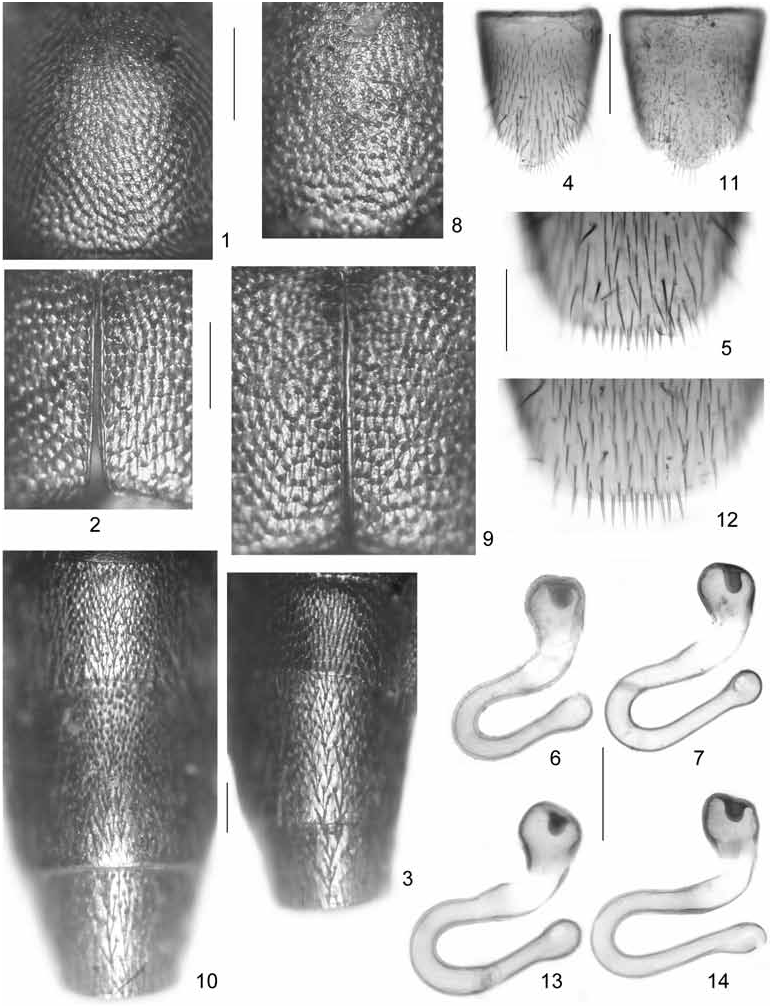
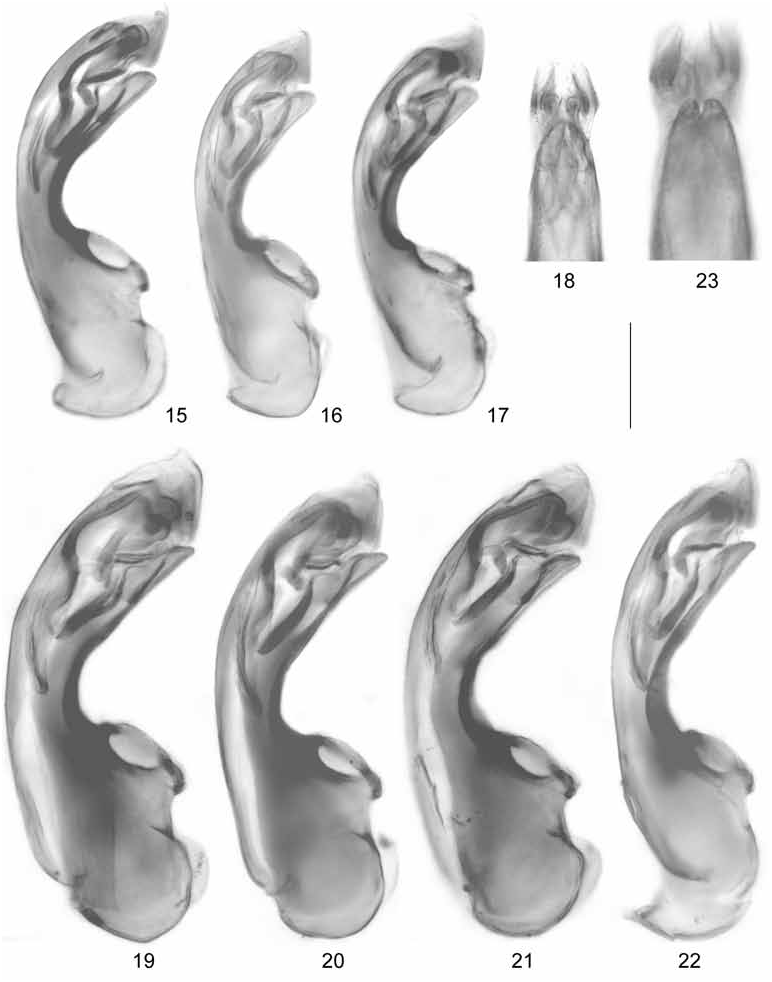
Oxypoda brachyptera ( STEPHENS, 1832) View in CoL ( Figs 1-7 View Figs 1-14 , 15-18 View Figs 15-23 , 24-27 View Fig View Fig View Fig View Fig )
Aleochara brachyptera Stephens, 1832: 128 .
Oxypoda forticornis Fairmaire & Brisout de Barneville, 1859: 37 View in CoL f.
Bessopora subrugosa Sahlberg, 1876: 111 View in CoL f.
Oxypoda difficilis Roubal, 1931: 70 View in CoL f.
Oxypoda maritima Donisthorpe, 1932a: 3 View in CoL .
Oxypoda salictaria Donisthorpe, 1932b: 4 View in CoL .
Oxypoda brachyptera View in CoL f. obscura Korge, 1959: 61.
Oxypoda brachyptera View in CoL f. wagneri Korge, 1959: 59 ff.
Type material examined:
Syntype : “h3 5/1 / 9 [6?] / Kirby / brachyptera / Syntype / Syntype Aleochara brachyptera Stephens from Kirby colln., det. R. G. Booth 2011 / Oxypoda brachyptera (Stephens) det. V. Assing 2011 ” ( BMNH) .
Comment:
The original description of Aleochara brachyptera is based on an unspecified number of syntypes from “ Norfolk ” and “Barham” ( Stephens 1832). From the punctuation code ( Hammond 1972) used by Stephens (1829) and the fact that the species was attributed to “Kirby MSS” by Stephens (1829, 1832), it can be inferred that the syntypes are not deposited in the Stephens collection, but in other collections. One female syntype was located in the Kirby collection. This syntype is conspecific with the previous interpretation of Oxypoda brachyptera .
Additional material examined:
Apart from the material listed in Tab. 2 View Tab , the following material was studied:
Germany: Niedersachsen: 1 [macropterous], Hannover, Osterfelddamm , pitfall, VIII-IX.1991, leg. Sprick (cAss); 1 , 2 [micropterous], Hannover-Herrenhausen, garden, pitfall, IV.1986 (cAss); 1 [micropterous], same data, but VI.1985 (cAss); 2 , Hannover-Langenhagen, sandy grassland, pitfall, V.1991, leg. Sprick (cAss); 4 , 2 [micropterous], S Hannover Ronnenberg, potash mine, salt habitat, pitfall, VII.1995, leg. Schmidt (cAss); 1 [macropterous], Neustadt / Rbg., Himmelreich, window trap, VII.1991, leg. Assing (cAss); 1 [micropterous], Hameln env., Grossenwieden, field margin, pitfall, IV.1986, leg. Sprick (cAss); 2 [micropterous], Süntel, Rannenberg, calcareous arable land, pitfall, V.1988, leg. Sprick (cAss); 1 [micropterous], Göttingen env., Fredelsloh, calcareous grassland, pitfall, VI.1984, leg. Joger (cAss); 1 [micropterous], Bückeburg env., Ahnsen, arable land, pitfall, V.1986, leg. Sprick (cAss); 1 [micropterous], 1 [macropterous], same data, VI.1986 (cAss); 1 [micropterous], Braunschweig, arable land, V.1988 (cAss); 1 [macropterous], Wilhelmshaven, Neuenburger Urwald, window trap, VIII.1996, leg. Menke (cAss) . Schleswig-Holstein: 124 exs. [11 macropterous], Husum env., Beltringharder Koog [54°33'N, 8°55'E], salt marsh, meadow, pitfall, VI-X.1991 (cAss) GoogleMaps . Sachsen: 2 [macropterous], Leipzig , uncultivated arable land, pitfall, V.1995, leg. Sprick (cAss); 1 [macropterous], same data, but V-VII.1995 (cAss); 1 , Leipziger Auwald NSG Burgaue, window trap, 1.VII.2003 (cSch) GoogleMaps .
Austria: Kärnten: 1 [micropterous], Gurktaler Alpen, Innerkrems, Gaipahöhe , 2100-2150 m, N-slope, 17.VII.1986, leg. Assing (cAss) .
Redescription:
Small species; body length 2.2-2.6 mm. Coloration variable; usual coloration: head reddishbrown to blackish-brown; pronotum and elytra reddish-yellow to reddish; abdomen reddish, with segment VI and anterior portion of segment VII infuscate; legs yellowish to reddish-yellow; antennae dark-yellowish to brown. Occasionally, especially in macropterous specimens, the coloration is significantly darker, with the head almost blackish, the pronotum and elytra dark-brown, and the abdomen more extensively infuscate.
Head transverse; eyes moderately large, approximately as long as postocular portion in lateral view, not larger in macropterous than in micropterous specimens. Antenna relatively long and massive, moderately and gradually incrassate apically; preapical antennomeres approximately 1.5 times as wide as long; antennomere XI with weakly pronounced sexual dimorphism, on average slightly longer in males than in females. Maxillary palpus not conspicuously elongated; preapical palpomere approximately 3 times as long as wide.
Pronotum approximately 1.35 times as broad as long, widest approximately in the middle; hind margin broadly convex, not distinctly bisinuate; punctation dense and shallow; interstices with microsculpture ( Fig. 1 View Figs 1-14 ).
Elytra dimorphic, in micropterous morph 0.7-0.8 times, in macropterous morph 0.90-0.95 times as long as pronotum; posterior margin distinctly sinuate near postero-lateral angles; punctation dense, usually somewhat coarser than that of pronotum ( Fig. 2 View Figs 1-14 ). Hind wings dimorphic, either fully developed (macropterous morph) or reduced to short stubs (micropterous morph); exceptionally (only one specimen seen) submacropterous. Metatarsomere I almost as long as combined length of metatarsomeres II-IV.
Abdomen with segments III-VI of subequal width; segments VII-X tapering. Punctation fine, dense on tergites III-VI, somewhat sparser on posterior tergites ( Fig. 3 View Figs 1-14 ); posterior margin of tergite VII with palisade fringe in both morphs; posterior margin of tergite VIII weakly convex.
: sternite VIII produced posteriorly ( Fig. 4 View Figs 1-14 ); aedeagus with median lobe 0.30-0.35 mm long ( Fig. 15-17 View Figs 15-23 ); ventral process apically incised in ventral view ( Fig. 18 View Figs 15-23 ); paramere with long apical lobe.
: sternite VIII with broadly convex posterior margin, with row of modified, stouter marginal setae ( Fig. 5 View Figs 1-14 ); spermatheca as in Figs 6-7 View Figs 1-14 .
Systematic position:
Oxypoda brachyptera is currently placed in the subgenus Bessopora Thomson, 1859 (type species: Oxyopda testacea Erichson, 1837).
Comparative notes:
In Central Europe, the only species of similarly small size, similar coloration, body shape, punctation, a pronounced pterodimorphism, and similar sexual characters is O. tarda . For notes on the separation of O. brachyptera from this species see the following section. In external morphology, the macropterous morph of O. brachyptera also somewhat resembles O. ferruginea Erichson, 1839 , with which it has had a history of confusion ( Horion 1967). For illustrations of the sexual characters of this species see Assing (2011). From similar Mediterranean representatives of the O. brachyptera group and of other species groups, such as O. caespita Assing, 2003 , O. lesbia Assing, 2005 , O. ahirica Assing, 2006 , O. afimbriata Assing, 2006 , O. praecisa Assing, 2006 , and O. cingulum Bernhauer, 1902 , O. brachyptera is separated by the combination of larger eyes, relatively longer elytra (even in the micropterous morph), the presence of a pterodimorphism, as well as by the sexual characters. For illustrations of the compared species see Assing (2003, 2005, 2006a, 2006b). For more images of the habitus, as well as of the primary and secondary sexual characters of O. brachyptera see Klimaszewski et al. (2006).
Distribution:
According to Horion (1967) and Smetana (2004), O. brachyptera is distributed in the North Palaearctic from Italy, France, and the British Isles across Central Europe eastwards to East Siberia and the Russian Far East. However, in view of frequent previous confusion with other similar Oxypoda species , the distribution requires revision. So far, I have seen true O. brachyptera only from Central Europe and Great Britain. Klimaszewski et al. (2006) report the species from Canada, where it is probably adventive.
Natural history:
Habitat. In northern Germany, O. brachyptera occurs in more or less unshaded lowland habitats on well-drained, sandy or calcareous soils ( Tab. 2 View Tab ). It is particularly abundant in dry, xerothermous Calluna heathlands and in grasslands with either sparse or low vegetation cover (early succession stages of sandy habitats, lawns). The species was not recorded in dense forests, in moist grass- or heathland, and on heavy soils. In the pitfall transsect in the study site Rössenbergheide- Külsenmoor, it was recorded in greater numbers in the drier Calluna slopes, whereas it was absent from the lower Erica heathland ( Fig. 24 View Fig ). One specimen was collected in a subalpine habitat in the Alp, at an altitude of 2100-2150 (see additional material examined). In view of the frequent confusion with other species, particularly O. tarda and O. ferruginea , possibly also the similarly coloured and similarly small O. exoleta Erichson, 1839 , literature data on the ecology of O. brachyptera are mostly unreliable. Horion (1967), for instance, reports the species from both moist soils, shores, and banks, and from dry sandy soils.
Phenology:
Adult O. brachyptera were recorded with pitfall traps from the beginning of March to the beginning of December. However, epigeic activity is low to very low in March and from September through the first half of December and probably only occurs when the weather conditions are favourable. The core activity period lasts from April through August, with a conspicuous peak in the second half of April and two less evident maxima in the second half of June and in the second half of August ( Fig. 25 View Fig ).
In some study sites, especially Heiliger Hain and Schneverdingen, O. brachyptera was among the most abundant staphylinid species in the pitfall traps, despite its small body size. As can be seen in Fig. 26 View Fig , the pooled seasonal densities are relatively low. They are highest in spring and range between less than one to approximately 4.5 individuals per square meter.Thus, it can be concluded that, in comparison to other staphylinids of similar and even larger body size, the epigeic activity of O. brachyptera from April through August is enormous. The sex ratio (males:females) in the soil samples was 0.62, whereas in the pitfall traps it was 1.24, suggesting that the epigeic activity of males is distinctly greater than that of females. This particularly applies to the period from the second half of April through the first half of August, during which time the proportions of males in the pitfall traps ranged between 55.5 and 61.6 %.
As can be inferred from the data shown in Fig. 27 View Fig , O. brachyptera apparently has two generations per year. Oviposition takes place from the second half of March to the second half of September. However, there are two maxima, one from mid-April to mid-May and one from Mid-June to mid-August. Emergence from the pupa occurs from the beginning of May to the first half of December, again with two maxima. The first one is from the beginning of June to mid-July and the second one in the second half of August. A comparison of the two curves in Fig. 27 View Fig suggests that pre-imaginal development from oviposition to emergence from the pupa lasts approximately 1.5-2 months and that hibernation occurs in the adult stage. There appears to be no aestivation period or diapause.
On one occasion (Hannover, August), one dissected female was found to be infested with nematodes.
Pterodimorphism and dispersal:
The wing dimorphism in O. brachyptera is not sex-related; the proportions of both morphs were similar for both sexes. In all the study plots, the vast majority of specimens was micropterous. The macropterous morph made up only some 2.4 % of the grand total. The proportion of macropterous specimens was highest in sandy habitats in early succession stages, in urban habitats (lawns, sandy grassland) ( Tab. 2 View Tab ), on arable land, and in a coastal meadow. Approximately 85 % of the dissected macropterous specimens had fully developed flight muscles. It is uncertain if the absence of flight muscles in the remaining 15 % is a result of post-mortem decay, genetic disposition, or metabolic reduction. In any case, the data suggest that at least the vast majority of macropterous individuals is capable of flight. On three occasions, flying specimens were recorded with window traps in July and August.
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Oxypoda brachyptera ( STEPHENS , 1832)
| Assing, Volker 2012 |
Oxypoda brachyptera
| Korge, H. 1959: 61 |
Oxypoda brachyptera
| Korge, H. 1959: 59 |
Oxypoda maritima
| Donisthorpe, H. 1932: 3 |
Oxypoda salictaria
| Donisthorpe, H. 1932: 4 |
Oxypoda difficilis
| Roubal, J. 1931: 70 |
Bessopora subrugosa
| Sahlberg, J. R. 1876: 111 |
Oxypoda forticornis
| Fairmaire, L. & Brisout de Barneville, C. 1859: 37 |
Aleochara brachyptera
| Stephens, J. F. 1832: 128 |