Neoromicia nanus (Peters, 1852)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6397752 |
|
DOI |
https://doi.org/10.5281/zenodo.6558731 |
|
persistent identifier |
https://treatment.plazi.org/id/4C3D87E8-FFC4-6A04-FA82-93C11ACBB1B7 |
|
treatment provided by |
Conny |
|
scientific name |
Neoromicia nanus |
| status |
|
126. View Plate 60: Vespe
Banana Serotine
French: Vespére naine / German: Bananen-Zwergfledermaus / Spanish: Neoromicia enana
Other common names: Banana Bat, Banana Pipistrelle, Banana Pipistrelle Bat
Taxonomy. Vespertilio nanus Peters, 1852 View in CoL ,
Inhambane, Mozambique.
Often treated in Pipistrellus , sometimes in a subgenus (typically Hypsugo or Pipistrellus ); due to its nyctalodont lower molars. This species might include a complex and clearly needs revision. Seven subspecies recognized.
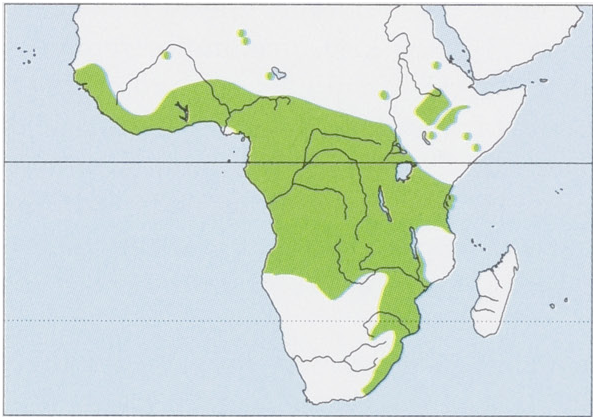
Subspecies and Distribution.
N.n.nanusPeters,1852—DRCongo,Kenya,andTanzaniaStoSouthAfrica;alsoinCameroon.
N.n.africanaRuppell,1842—EthiopiaStoDRCongo,mainlyinthehighlands.
N.n.culexThomas,1911—GhanaandNigeria.
N.n.fourieiThomas,1926—SAngola,WZambia,andNNamibia.
N.n.meester:Kock,2001—formerTranskei,ESouthAfrica.
N.n.minusculaG.S.Miller,1900—Liberia.
N. n. stampfliiJentink, 1888 — Sierra Leone to Ivory Coast.
The assignment of specimens from Sudan, South Sudan, and Somalia is uncertain. Also present in Senegal, Mali, and Niger, subspecies unknown. View Figure
Descriptive notes. Head-body ¢.40-44 mm, tail 24-40 mm, ear 7-13 mm, hindfoot 5-9 mm, forearm 25-35 mm; weight 2-5-6-5 g. Females average larger than males. Size increases from Sierra Leone to Cameroon. Pelage of the Banana Serotine is soft, dense, and fairy fluffy, with silky sheen; dorsally chocolate-brown or sepia, usually with golden sheen (hairs with basal one-half blackish brown, terminal one-half chocolate or sepia, usually golden brown at tip; mid-dorsal hairs ¢. 5 mm long); ventrally dark grayish buff (hairs blackish brown at base, grayish buff at tip). Wings are blackish brown, without white hind border; interfemoral membrane is blackish brown, sometimes with posterior margin pale or white. Ears are blackish brown, short compared to congeners, subtriangular, and with rounded tip; outer margin has lobule at base inconspicuous or missing; tragus is about one-half ear length;it is broadest well above mid-height, and hatched-shaped, with anterior margin slightly or distinctly concave; posterior margin forms obtuse-angle bend just above mid-height; no basal lobe; tip is narrow and rounded. Thumb and hindfoot are very small, with friction pad on wrist and sole. Skull very variable in size, but small compared to other African pipistrelle-like bats (greatest skull lengths 10-12-6 mm); braincase is normally high and of medium relative breadth; interorbital region has medium relative breadth; rostrum is on average relatively short and narrow; and profile of forehead is strongly concave, without occipital helmet. I? is usually bicuspid, sometimes unicuspid; I is at least one-half height of I?, and often reaches posterior cusp of I?; P* is medium-sized, somewhat lingually displaced; C! and P* are not in contact; lower molars are nyctalodont. Dental formulais12/3,C1/1,P 2/2, M 3/3 (x2) = 34. Chromosomal complement has 2n = 36 and FNa = 50. Data from Senegal of 2n = 34, FNa = 50, and FN = 54 suggest that may be taxonomically distinct.
Habitat. Diverse forest and savanna habitats, and desert steppe, from sea level to 2500 m. The Banana Serotine may depend on musaceous plants for roosts, being apparently restricted to habitats warm and moist enough to allow these plants to grow.
Food and Feeding. In Malawi, forages by slow hawking in moderately uncluttered places such as clearings, gaps between trees, and around tree canopies. It forages acrobatically. Prey volume percentages at Umbilo River, eastern South Africa, during summer were (mean + SD): Diptera (43:6 + 17-4), Hemiptera (34-6 + 14-1), Trichoptera (19-6 + 6-8), and Lepidoptera (2-3 + 1-4); and during winter: Lepidoptera (36-4 + 2.5), Diptera (29:5 = 3-7), Hemiptera (19:1 = 7-3), Trichoptera (12 + 7-2), and Coleoptera (3-2 = 0-9).
Breeding. Littersize is invariably two in Zimbabwe, Malawi, and Zambia; females with one or two embryos have been recorded from South Africa, Namibia, DR Congo, Gabon, Ghana, and Liberia (females with only one young might have given birth to twins and lost one). Thought to be seasonal monoestrous. In Mpumalanga Province, northeastern South Africa, spermatogenesis began in late September and spermatozoa were present in the caudae epididymides from late April through to August. Mating started in May and ovulation and fertilization occurred from early August, concomitantly with the first implantations. Parturition occurred from late October to late November. Lactating females were found until mid-January, c.1 month earlier than in Malawi. Five lactating females were captured at the end of November at Uzungwa Scarp Forest Reserve, east-central Tanzania. Mating groups consist of a single male and several females, but cannot be considered as harems. Neonates are naked, eyes closed, and weigh c. 1 g; detached neonates make vocalizations but do not appear to echolocate; in third week, they echolocate and are capable of efficient cursorial scuttling over horizontal and sloping surfaces; in seventh week, deciduous dentition begins to be replaced; in ninth week, diet already includes insects but also milk, and young are just volant; weaning occurs at ¢.8-9 weeks. Sex ratio at birth is 1:1.
Activity patterns. Foraging begins at dusk and individuals return to their furled leaves just before sunrise. The Banana Serotine is most active in the first five hours after sunset, activity declining rapidly after midnight. It roosts by day in furled leaves of domestic bananas and plantain plants of Musa spp. , wild bananas Ensete (both Musaceae ), and Strelitzia nicolai ( Strelitziaceae ). Individuals become torpid during the day during the cool, dry season; in hot, wet season, only a few become torpid. Aspect ratio is low, wing loading very low, and flight slow and high maneuverable in confined spaces, faster and very agile in open spaces; bats can take off from ground, and are moderately efficient in cursorial locomotion on ground, and climbing on sloping surfaces;friction pads present on the wrists and hindfeetfacilitate climbing on banana leaves. Aggregated data from Swaziland and Durban give: maximum frequencies of 74-4-80-9 kHz, minimum frequencies 64-9-71-8 kHz, frequencies of the knee 66-1-75-1 kHz, characteristic frequencies 65-4-72-2 kHz, and durations 2-4-3-6 milliseconds. In western Uganda (mean + SD): minimum frequency of 71-1 + 6 kHz, maximum frequency 98-9 + 4-8 kHz, characteristic frequency 79-8 + 8-2 kHz, frequency of the knee 84-9 + 7-1 kHz, and duration 1-1 + 0-1 milliseconds. In Aberdare Range in Kenya, peak frequencies of 63-69-9 kHz, maximum frequencies 69:6—-117-9 kHz, and minimum frequencies 59-8-66-9 kHz. Potential predators include the opisthoglyphe savanna vine snake (Thelotornis capensis ) and the bat hawk (Macheiramphus alcinus); predation by a grey-headed bush-shrike (Malaconotus blanchoti) was observed; and one bat was found dead in a spider web.
Movements, Home range and Social organization. The Banana Serotine has a relatively small home range of ¢. 300 m around the roost. Where separate banana plantations may be apart, individuals may maintain at least two roost ranges, and alternate between them. In South Africa, adult males have been reported to roost singly or in labile groups of 1-6 adult males and 1-8 adult females without young. About one-third of banded bats are recaptured, suggesting they may be sedentary. The species usesterritorial calls, possibly with a courtship function, consisting of a simple syllable-type in series.
Status and Conservation. Classified as Least Concern on The IUCNRed List (as N. nana). Due to its use of banana leaves for day roosts,this is one of the few species that is benefited with the conversion of natural habitats into subsistence farms and villages.
Bibliography. ACR (2017), Bernard et al. (1997), Coghlan (2001), Eisenring et al. (2016), Happold, D.C.D. (2001), Happold, D.C.D. & Happold (1988, 1996), Happold, M. (2013r), Hill, J.E.& Harrison (1987), Hill, K.et al. (2016), ICZN (2005), Kearney et al. (2002), Keeley & Keeley (2004), Koopman (1965, 1975, 1993), Koubinova et al. (2013), LaVal & LaVal (1977), McCracken & Wilkinson (2000), Menu (1987), van der Merwe & Stirnemann(2007), Monadjem, Ellstrom et al. (2010), Monadjem, Rasmussen & van der Made (2011), Monadjem, Shapiro et al. (2017), Naidoo, Mackey & Schoeman (2011), Manga Mongombe (2012), Naidoo, Schoeman etal. (2009), Naidoo, Vosloo & Schoeman (2013a, 2013b, 2015, 2016), Perret & Aellen (1956), Peterson (1987), Peterson & Nagorsen (1975), Rautenbach et al. (1993), Rosevear (1965), Ripell (1842), Schoeman & Waddington (2011), Simmons (2005), Taylor, Monadjem & Steyn (2013), Taylor, Sowler et al. (2013), Trentin & Rovero (2011), Volleth et al. (2001).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Neoromicia nanus
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio nanus
| Peters 1852 |