Osteocephalus castaneicola, Moravec, Ji Ř Í, Aparicio, James, Guerrero-Reinhard, Marcelo, Calderón, Gonzalo, Jungfer, Karl-Heinz & Gvoždík, Václav, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.189932 |
|
DOI |
https://doi.org/10.5281/zenodo.5621756 |
|
persistent identifier |
https://treatment.plazi.org/id/495587FB-DD51-E134-FF0B-FA7C19D3D5E9 |
|
treatment provided by |
Plazi |
|
scientific name |
Osteocephalus castaneicola |
| status |
sp. nov. |
Osteocephalus castaneicola View in CoL sp. n.
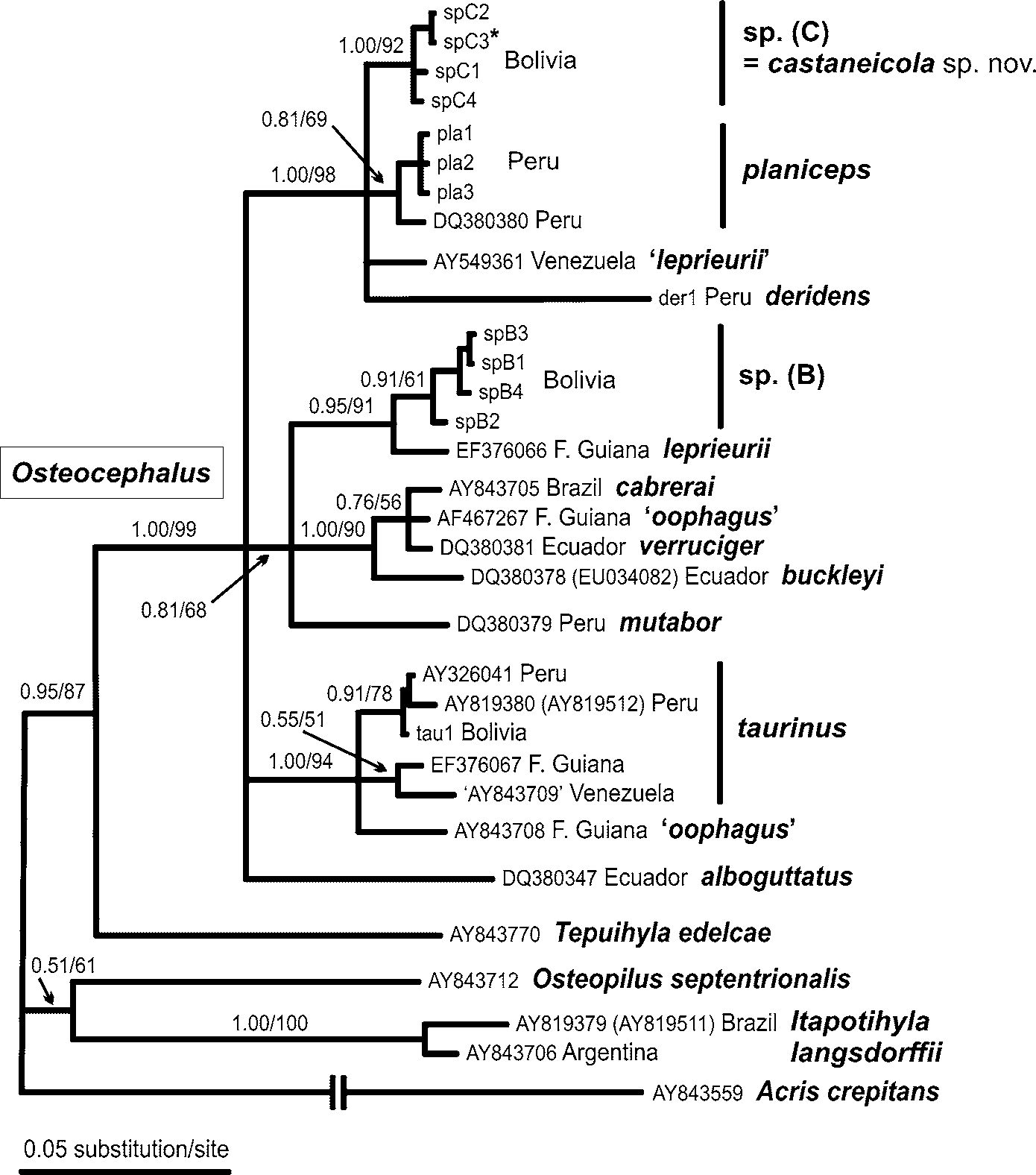
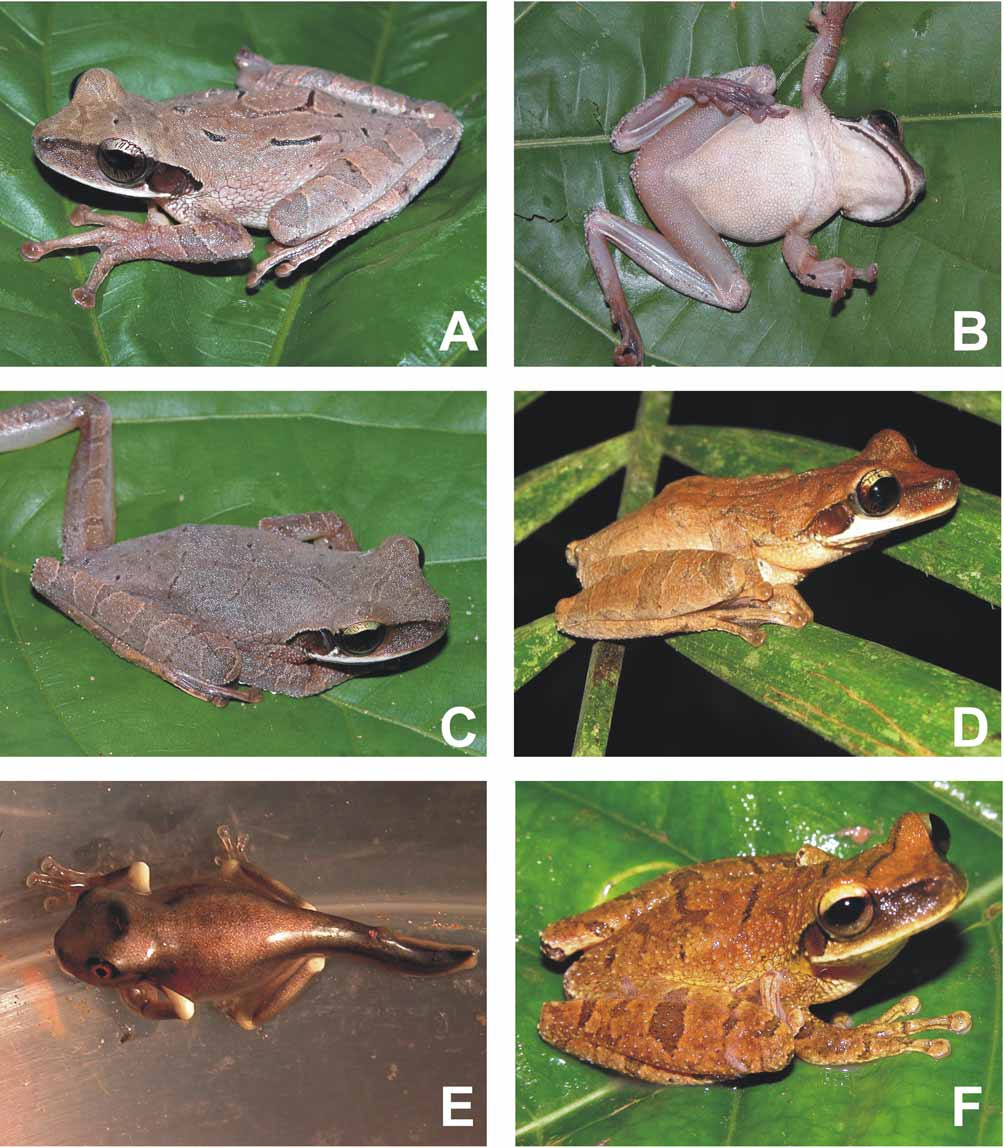
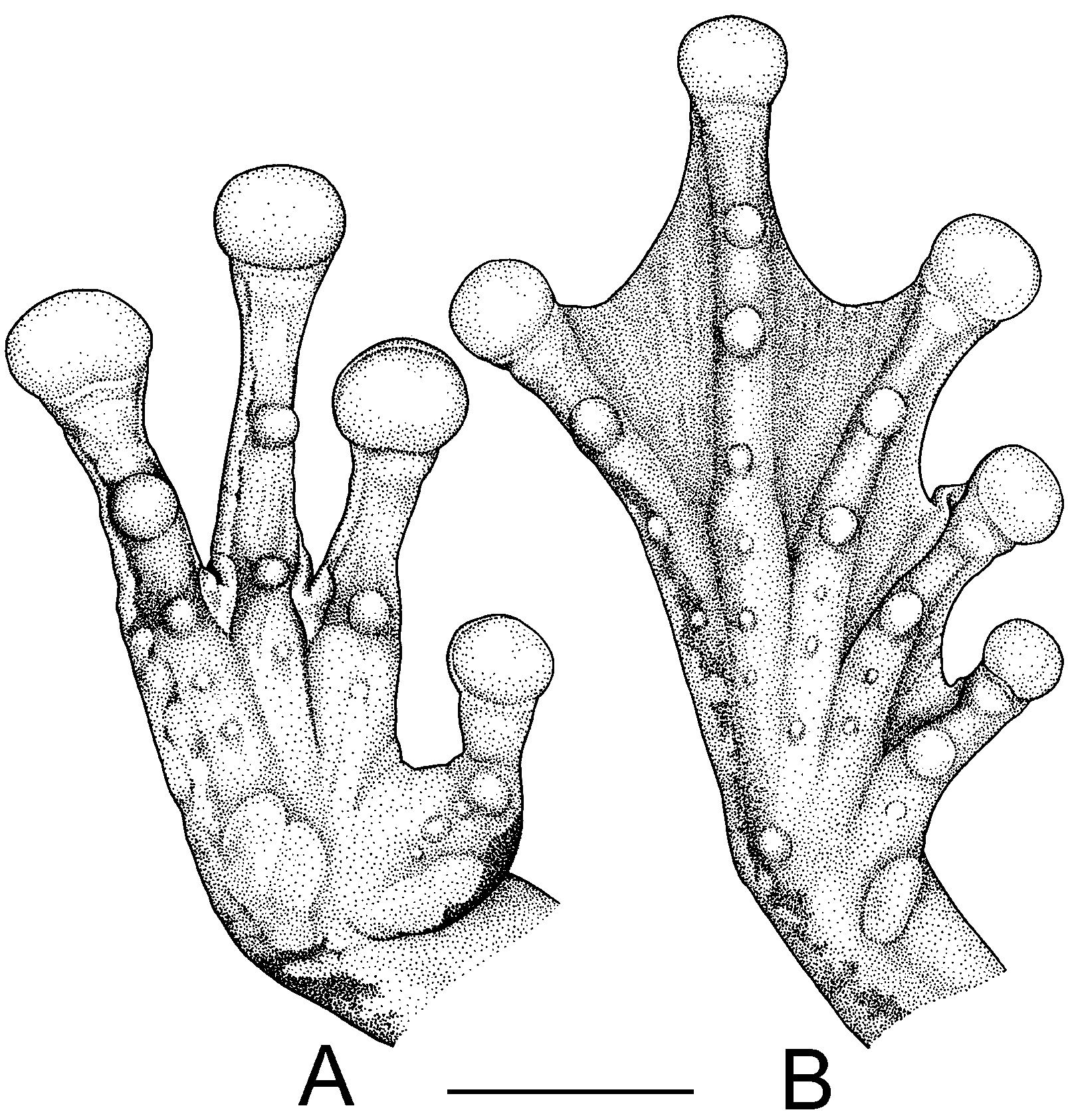
Figs. 2 View FIGURE 2 (A–E), 3(A–B)
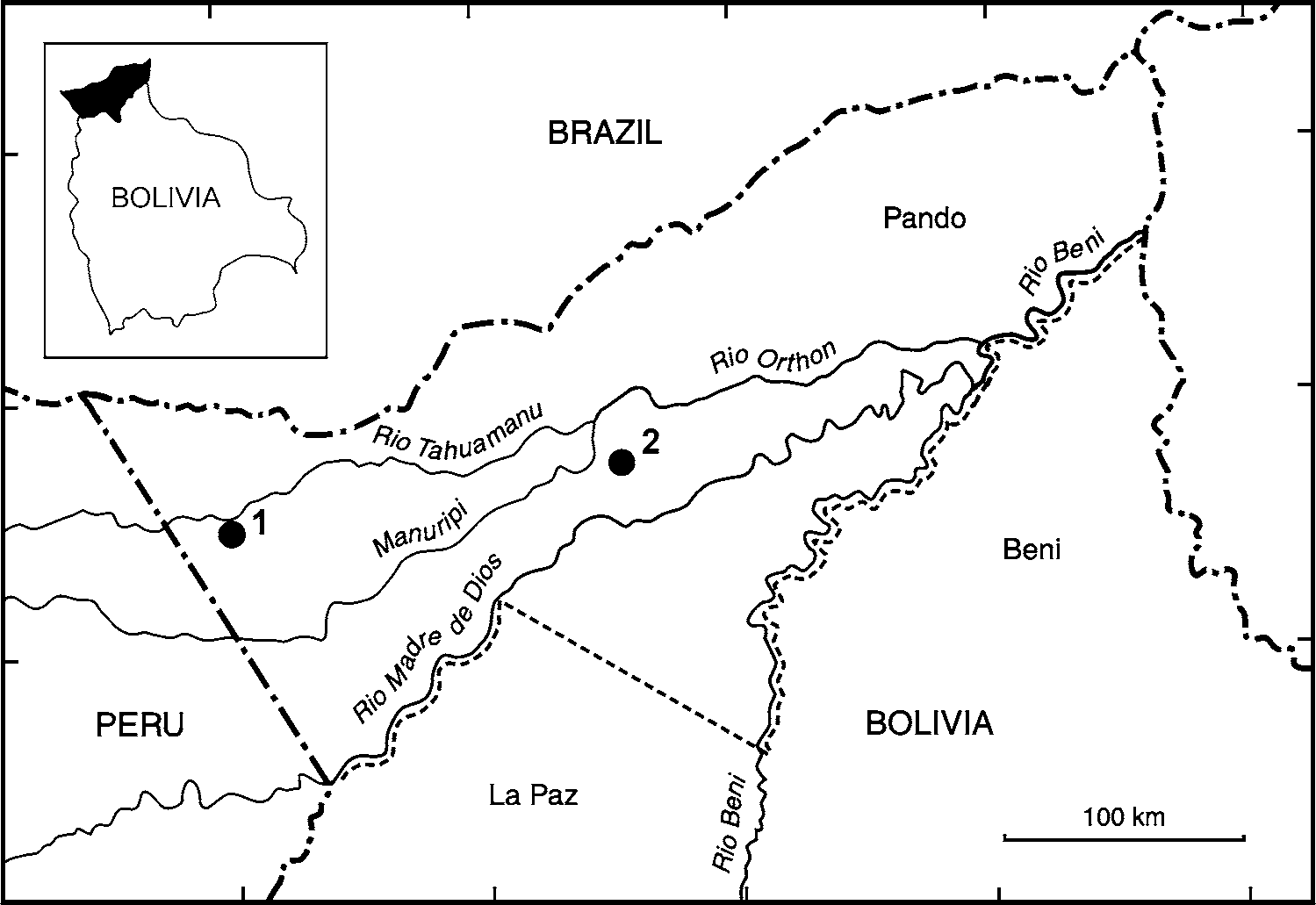
Holotype. CBF 6051, adult male from the vicinity of the settlement of San Antonio de Filadelfia, 11°18’ S, 67°23’ W, ca. 200 m a.s.l., Provincia Manuripi, Departamento Pando, Bolivia, collected on 22 November 2007 by J. Moravec, M. Guerrero-Reinhard and G. Calderón.
Paratopotypes. NMP 6 V View Materials 73810/1–3, two adult males and an adult female, same locality and collecting data as holotype; CBF 6052, adult female, same locality and collecting data as holotype;
Paratypes. CBF 6053–6054, adult male and adult female from San Antonio del Matti, 11°30’S, 68°53’W, ca. 270 m a.s.l., Provincia Manuripi, Departamento Pando, Bolivia, collected on 27 November 2007 by J. Moravec, M. Guerrero-Reinhard and G. Calderón; NMP 6 V View Materials 73820, adult female, same locality and collecting data as CBF 6053–6054.
Diagnosis. A medium-sized species of Osteocephalus as revealed from mtDNA analyses, which can be distinguished by the following combination of characters: (1) medium size, SVL 47.8–51.3 mm in males, 47.7–63.3 mm in females; (2) snout rounded in dorsal view, rounded and slightly inclined posteroventrally in lateral view; (3) canthus rostralis distinct, angular, distinctly curved medially; loreal region concave; (4) low frontoparietal ridges well-marked in large individuals; (5) tympanum large, round to oval, about 62.5–76.5% of eye diameter, tympanic annulus distinct; supratympanic fold markedly developed; (6) vocal slits absent, vocal sac indistinct; (7) vomerine odontophores large, prominent, angular, narrowly separated or in contact medially, between oblique choanae, bearing 6–14 vomerine teeth each; (8) skin on dorsal surfaces with numerous minute tubercles; (9) low tarsal and ulnar tubercles present, slightly larger than dorsal tubercles; (10) axillary membrane absent; (11) basal webbing on hand [webbing formula II (2– –2+)—(3– –3+) III (3– –3)— (2 2/3–3–) IV]; toes about three fourths webbed [webbing formula I (1–1 1/4)—(1 2/3–2–) II (1–1+)—(2– –2) III (1– 1+)—(1 2/3–2) IV (1 2/3–2–)—(1– –1) V]; (12) single round distal subarticular tubercle under the fourth finger; (13) dark keratinous excrescences restricted to prepollex; (14) in life, dorsum tan, pale brown to purple brown, with scarce narrow irregular dark brown markings; a narrow pale supralabial line expanding in a subocular spot; flanks pale, without markings; hidden surfaces of thighs light brown; throat and belly creamy white; a narrow dark line along the mandible; ventral surfaces of thighs fleshy pink; iris bicoloured with a dark horizontal stripe, golden above, bronze below, both parts with fine dark reticulate to radiate lines; tibiae green or white; (15) in life, newly metamorphosed juveniles light brown dorsally, with a dark interorbital spot, bright orange iris, and creamy white upper arms, knees and heels.
Comparisons. Morphologically, O. castaneicola can be distinguished from all other Amazonian species of Osteocephalus by absence of vocal slits and by the following combinations of characters: from O. alboguttatus by more extensive webbing and by colouration ( O. alboguttatus : toes two thirds webbed, light brown dorsum with small blackish dots, flanks and upper surface of thighs with small round white spots, beneath whitish with dark reticulation) ( Boulenger 1882, Duellman 1978); from O. buckleyi by absence of large tarsal tubercles, absence of patagium and by eye colouration ( O. buckleyi : large tubercles along the tarsus, well developed patagium, light iris without conspicuous dark pattern) ( Boulenger 1882, Cochran & Goin 1970; examined specimens listed in the Appendix); from O. cabrerai by absence of large dorsal, ulnar and tarsal tubercles, absence of patagium and by colouration ( O. cabrerai : large wart-like tubercles on head and dorsum, large tubercles along the ulna and tarsus, small patagium, irregularly mottled dorsal pattern, light iris with very fine vermiculation) ( Cochran & Goin 1970; examined specimens listed in the Appendix); from O. carri (Cochran & Goin) by colouration ( O. carri : dense large irregular dark spots on the dorsum, black spots on flanks, fuscous throat and chest) ( Cochran & Goin 1970); from O. deridens by larger size and by colouration ( O. deridens : SVL up to 34.9 mm in males and 50.6 mm in females, dorsum light or dark tan with or without irregular darker or lighter markings, golden yellow iris with a dark horizontal stripe and regular dark radiation ( Jungfer et al. 2000; examined specimens listed in the Appendix); from O. elkejungingerae (Henle) by skin texture and by colouration ( O. elkejungingerae : conspicuous tubercles with keratinized tips in breeding males, dorsum with broad light dorsolateral stripes in juvenile and subadult specimens (Henle et al. 1983; Jungfer et al. 2000; examined specimens listed in the Appendix); from O. fuscifacies by larger size and by colouration ( O. fuscifacies : SVL up to 45.6 mm in males and 53.2 in females, dorsum light or dark tan with or without irregular darker or lighter markings, light subocular spot absent, venter dark with creamy white granules or creamy white, golden iris with a dark horizontal stripe and regular dark radiation ( Jungfer et al. 2000; examined specimens listed in the Appendix); from O. heyeri Lynch by larger size and by colouration ( O. heyeri : SVL up to 36.1 mm in males and 47.7 mm in females, dorsum brown with darker markings and pale spots, flanks with pale spots, hidden surfaces of limbs dark brown with pale spots, iris dark) ( Lynch 2002); from O. leoninae Jungfer & Lehr by larger size and by colouration ( O. leoninae : SVL up to 42.0 mm in males and 53.2 mm in females, upper part of iris yellow without dark markings, unpigmented nuptial pads, bold dorsal pattern) ( Jungfer & Lehr 2001, Chávez et al. 2008); from O. leprieurii by nuptial excrescences restricted to prepollex, skin texture and by colouration ( O. leprieurii : prepollical and subdigital nuptial excrescences, numerous conspicuous tubercles with keratinized tips in breeding males, golden iris with dark vermiculation, white supralabial stripe in juveniles) ( Jungfer & Hödl 2002); from O. mutabor by skin texture and by colouration ( O. mutabor : numerous conspicuous tubercles with keratinized tips in breeding males, bold dark transverse markings, golden yellow iris with dark vermiculation, white dorsolateral stripes in juveniles) ( Jungfer & Hödl 2002; examined specimens listed in the Appendix); from O. oophagus by head shape and by colouration ( O. oophagus : truncate snout in dorsal view, white mottling or reticulation on posterior half of the flanks, golden iris with regular black radiation, orange spots on elbow, knee and heel in juveniles) ( Jungfer & Schiesari 1995; examined specimens listed in the Appendix); from O. pearsoni by skin texture and by colouration ( O. pearsoni : small nonspinous tubercles in males, black reticulation on the venter, dark iris) ( Trueb & Duellman 1971, Jungfer & Schiesari 1995, Jungfer & Lehr 2001); from O. planiceps by smaller size, skin texture, keratinous excrescences restricted on prepollex and by colouration ( O. planiceps : SVL up to 65.9 mm in males and 88.2 mm in females, numerous conspicuous tubercles with keratinized tips in breeding males, keratinous excrescences extending laterally to disc of thumb, dark spots on flanks, iris with regular black radiation) ( Cope 1874, Duellman & Mendelson 1995, Jungfer & Lehr 2001, examined specimens listed in the Appendix); from O. subtilis Martins & Cardoso by larger size and by colouration ( O. subtilis : SVL up to 38.8 mm in males, dark iris) ( Martins & Cardoso 1987); from O. taurinus by smaller size, less webbing on the hands and by colouration ( O. taurinus : SVL up to 81.0 mm in males and 94.1 in females, fingers one-half webbed, dark spots on flanks, small brown flecks on the throat, chest and sides of the belly, greenish gold iris with regular black radiation) ( Duellman 2005; examined specimens listed in the Aappendix); from O. verruciger by skin texture and by colouration ( O. verruciger : numerous conspicuous tubercles with keratinized tips in breeding males, uniform reddish brown iris) ( Trueb & Duellman 1971, Jungfer et al. 2000, Jungfer & Hödl 2002); from O. yasuni by skin texture and by colouration ( O. yasuni : numerous conspicuous tubercles with keratinized tips in breeding males, yellow venter in adults, iris with irregular dark reticulation, intense yellow-orange venter and webbing in juveniles) ( Ron & Pramuk 1999, Jungfer et al. 2000, Jungfer & Hödl 2002, Cisneros-Heredia 2007).
There are seven available names in the synonymy of four Osteocephalus species: Hyla festae Perraca, 1904 (type locality: Ecuador: “Valle de Santiago” (= lower Río Zamora) Province of Morona-Santiago) in the synonymy of O. buckleyi ; Hyla leprieurii britti Melin, 1941 (type locality: Brazil: “Río Uaupés (north of the Río Japú”, Amazonas) and Osteocephalus ayarzaguenai Gorzula & Señaris, 1997 (type locality: Venezuela: “Campamento Airo, Valle del Río Karuay”, Estado Bolívar) in the synonymy of O. leprieurii ; Osteocephalus flavolineatus Steindachner, 1862 (type locality: Brazil: “Cocuy” (= Cucuí), Amazonas) and Hyla depressa Andersson, 1945 (type locality: Ecuador: “Río Pastaza, Watershed”) in the synonymy of O. taurinus ; and Hyla riopastazae Andersson, 1945 (type locality: Ecuador: “Baños, Río Pastaza, Provincia Tungurahua”) and Hyla orcesi Funkhouser, 1956 (type locality: Ecuador: “[Río] Pacayacu, a stream that flows into the Cotapino, drainage of the Suno, Río Napo region”) in the synonymy of O. verruciger . The new species differs from all of them by the following combination of characters: from Hyla festae by smaller size and by colouration (female holotype of H. festae : SVL 75.0 mm, large median longitudinal dark brown blotch on the dorsum, dark brown spots on flanks, throat and belly) ( Trueb & Duellman 1971); from Hyla leprieurii britti by nuptial excrescences restricted to prepollex and by skin texture (male holotype of H. l. britti : prepollical and subdigital nuptial excrescences and tuberculate dorsum) ( Trueb & Duellman 1971, Jungfer & Hödl 2002), from Osteocephalus ayarzaguenai by colouration ( O. ayarzaguenai : golden iris with dark vermiculation) ( Jungfer & Hödl 2002; examined specimen listed in the Appendix); from Osteocephalus flavolineatus by smaller size and colouration (female holotype of O. flavolineatus : SVL 81.8 mm, light middorsal stripe, spots on the flanks) ( Cochran & Goin 1970, Trueb & Duellman 1971); from Hyla depressa by smaller size, skin texture, and by colouration (male holotype of H. depressa : SVL 68.9 mm, tuberculate dorsum, light middorsal stripe) ( Cochran & Goin 1970, Trueb & Duellman 1971); from Hyla riopastazae by colouration ( H. riopastazae : brown spots and mottling on throat, chest and belly) ( Trueb & Duellman 1971); and from Hyla orcesi by skin texture and by colouration ( H. orcesi : tuberculate dorsum, ventral surfaces dirty brown) ( Cochran & Goin 1970, Trueb & Duellman 1971).
Description of the holotype. Adult male 51.3 mm SVL. Head narrower than body, slightly longer than wide; snout rounded in dorsal view, moderately protruding in lateral view; distance from nostril to eye shorter than diameter of eye; canthus rostralis distinct, angular, curved medially; loreal region concave; internarial area slightly depressed; nostrils moderately protuberant, directed laterally; interorbital area flat, IOD 112.2% of ELW; lateral margins of the frontoparietals barely visible through skin; eye large, strongly protuberant, its diameter about five times depth of lip below eye; tympanic membrane clearly evident, large, slightly wider than high, about two third of eye length, separated from eye by ca. 50% of its diameter; tympanic annulus distinct; supratympanic fold conspicuous, covering upper edge of tympanum, continuing above insertion of arm. Arm slender, axillary membrane absent; small low tubercles scattered along ventrolateral edge of forearm; relative length of fingers I<II<IV<III; fingers bearing large, oval discs, that of third finger about half of tympanum diameter; subarticular tubercles prominent, round, single; supernumerary tubercles present; palmar tubercle large, flat, disunited distally; prepollical tubercle large, flat, elliptical; prepollex enlarged; large dark keratinous nuptial excrescences covering inner surface of prepollex up to subarticular tubercle of thumb ( Fig. 3 View FIGURE 3 ); webbing rudimentary between fingers I and II; webbing formula of fingers II2 –—3– III3 –— 3– IV. Legs moderately long, slender; heels overlapping when limbs flexed perpendicular to the axis of body; small raised tubercles on the outer edge of tibiotarsal articulation; small low tubercles scattered along the ventrolateral edge of foot; toes moderately long, bearing oval discs slightly smaller than those of fingers; relative length of toes I<II<V<III<IV; outer metatarsal tubercle distinct, small, round; inner metatarsal tubercle large, ovoid; subarticular tubercles single, round, protuberant; supernumerary tubercles present; toes three fourths webbed; webbing formula of toes I1 +— 2–II 1— 2 –III1—2– IV2 –— 1–V. Skin on dorsum, head, and dorsal surfaces of limbs smooth, with numerous minute tubercles; skin on flanks shagreen; skin on venter coarsely granular; skin on throat slightly granular; proximal two thirds of lower surfaces of thighs slightly granular. Cloacal opening directed posteriorly at upper level of thighs; short simple cloacal sheath covering cloacal opening; rounded tubercles around vent and on posterior surface of proximal third of thigh. Tongue ovoid, widely attached to floor of mouth; vomerine odontophores angular, separated medially, between choanae, bearing 8 and 9 (left/right) vomerine teeth; choanae rhomboidal, oblique; vocal slits absent; vocal sac indistinct.
Measurements of the holotype: SVL 51.3; HL 17.7; HW 16.6; EN 5.3; ED 6.1; TD 4.0; ELW 4.9; IOD 5.4; TL 27.3; FL 33.4.
In alcohol, head and dorsum tan with several narrow irregular darker tan to dark brown markings (including an indistinct interorbital stripe) narrowly outlined by pale brown line; dorsal surfaces of limbs tan with darker tan crossbars outlined by a pale brown line. A narrow pale supralabial line expanding in a subocular spot; a dark canthal stripe extending from nostril to the anterior margin of eye; a broad dark brown postocular stripe extending from posterior margin of eye across the tympanum to insertion of arm. Flanks pale with several inconspicuous small darker markings; a dark supracloacal spot; hidden surfaces of thighs tan. Throat and belly creamy white; a narrow dark line along the lower jaw; ventral surfaces of thighs yellowish white; plantar surfaces pale brown. Tibiae green.
In life, dorsal and lateral colouration differed only slightly from the preserved specimen in having a slight purple-red tint by day. Ventral surfaces of forearms and thighs fleshy pink; tibiae green. Iris bicoloured with dark brown horizontal stripe, golden above, bronze below, both parts with fine dark reticulate to radiate lines ( Fig. 2 View FIGURE 2 A).
Variation. Variation of measurements of the type series is given in Table 3 View TABLE 3 . Osteocephalus castaneicola exhibits sexual dimorphism in body size, but sexual dimorphism of dorsal skin texture is absent. Both breeding males and females bear similar minute flat to round tubercles on dorsal surfaces of head, body and limbs. The most conspicuous dorsal tubercles are present in female paratopotype CBF 6052 ( Fig. 2 View FIGURE 2 C), having SVL 47.7 mm and containing numerous small immature eggs. The new species shows considerable variation in number of vomerine teeth (6–14 on each odontophore). Vomerine odontophores are separated in holotype, paratopotype NMP 6 V View Materials 72810/1 and paratypes CBF 6054 and NMP 6 V View Materials 73820, but in contact in the remaining types. Some variation seems to be evident in distinctiveness of lateral margins of the frontoparietals. They are not visible through skin in smaller individuals (SVL up to 47 mm; paratopotype CBF 6052 and paratype CBF 6053) and best pronounced in largest individuals (SVL above 59 mm; female paratopotype NMP 6 V View Materials 73810/3 and female paratypes CBF 6054 and NMP 6 V View Materials 73820). Some differences can be found in shape of distal subarticular tubercle of the fourth finger. It is single in holotype and four other type specimens, but it shows a slight tendency to bifidity in the paratopotype NMP 6 V View Materials 73810/3 and paratypes CBF 6053 and NMP 6 V View Materials 73820. The finger and toe webbing formulae vary as follows: II (2– –2+)—(3– –3+) III (3– –3)—(2 2/3–3–) IV and I (1–1 1/4) —(1 2/3–2–) II (1–1+)—(2– –2) III (1–1+)—(1 2/3–2) IV (1 2/3–2–)—(1– –1) V.
General dorsal colouration in alcohol varies from light tan to dark tan with purple-red tint or to reddishbrown. Dorsal pattern varies mostly regarding distinctness and shape of the irregular darker markings. A more or less distinct interorbital streak narrower than the diameter of the eye is present in all individuals. Dorsal markings are fused in a large, irregular, indistinct dorsal spot in the male paratype CBF 6053, whereas dorsal pattern of paratopotypes CBF 6052, NMP 6 V View Materials 73810/1, 73810/3 and paratype 73820 is almost missing. Ventral colouration in alcohol varies from cream white to yellowish-white. A fine dark brown mottling is present on the throat and pectoral area of the female paratype NMP 6 V View Materials 73820. Colour of tibiae seems to vary independently of age or size of individual specimens. The bones are green in the holotype and paratopotypes NMP 6 V View Materials 73810/1 – 3 (SVL 48.4–59.1 mm) and white in paratopotype CBF 6052 and paratypes CBF 6053, 6054 and NMP 6 V View Materials 73820 (SVL 47.7–63.3 mm).
In life, dorsal colouration varies from tan to brown. A slight purple-red tint observed in most specimens by day turns into ochre by night ( Fig. 2 View FIGURE 2 D). Newly metamorphosed juveniles are light brown dorsally with a dark interorbital spot, bright orange iris, and creamy white upper arms, knees and heels ( Fig. 2 View FIGURE 2 E).
Distribution, ecology and threat status. The known localities of Osteocephalus castaneicola lie in western and central part of the Departamento Pando, northern Bolivia ( Fig. 4 View FIGURE 4 ). This area is located in the south-western Amazon basin within the zone of tall evergreen lowland rainforest. O. castaneicola was encountered in more or less undisturbed terra firme forest with frequent occurrence of large climax forest trees [e.g. Bertholletia excelsa Humb. & Bonpl. , Ceiba pentandra (L.) Gaertn., Cedrela odorata L., Ficus sp.]. The forest was characterised by relatively well defined tree strata and a dense canopy at ca. 25–35 m above the ground. The understory was dominated by various tree seedlings, young trees, herbaceous lianas, palms and ferns. The forest floor was covered by leaf litter with scattered large fruit capsules of the Brazil nut tree ( Bertholletia excelsa ) and other species of Lecythiadaceae. All observed individuals of O. castaneicola were sitting on vegetation in ca. 0.5–2 m height. No calling males were located. Other hylid species found in sympatry with O. castaneicola included Hypsiboas lanciformis Cope , H. punctatus (Schneider) , Phyllomedusa camba De la Riva, P. tomopterna (Cope) , P. vaillantii Boulenger , Trachycephalus coriaceus (Peters) , and T. resinifictrix (Goeldi) . O. castaneicola is apparently known (as O. sp.) to occur also in the Region Madre de Dios (exact localities not provided) in adjacent southern Peru (von May et al. 2007).
Life history of O. castaneicola is closely associated with fruit capsules of the Brazil nut tree, which are opened by agoutis ( Dasyprocta sp.) or indigenous Brazil nut collectors and abandoned on the forest floor. At both known localities of O. castaneicola some of water-filled capsules contained tadpole assemblages numbering up to tens of individuals. Rarely the same tadpoles were found also in water-filled palm bracts lying on the ground. In some cases the assemblages consisted of larvae of markedly different sizes and different stage of development. The largest tadpoles reached a total length of 33–35 mm. Occasionally, white ingested eggs were visible through the transparent venter of the larger larvae. The tadpoles were raised until metamorphosis ( Fig. 2 View FIGURE 2 E) and their determination was verified by genetic comparison with the adult specimens ( Fig. 1 View FIGURE 1 ).
According to the sparse data available we here classify O. castaneicola as “Data Deficient” according to the IUCN red list criteria. In Peru, the species occurs within protected areas (von May et al. 2007).
Etymology. The specific name is a compound from the Latin castanea (Horse Chestnut, Aesculus ) from which the Spanish castaña (vernacular name of the Brazil nut tree) was derived and the Latin col ō (to inhabit). The name is used as a noun in apposition and refers to the life history of the new species.
TABLE 3. Variation of measurements (in mm) of the type series of Osteocephalus castaneicola sp. n.). See text for abbreviation.
| Measurement | Males (N=4) Mean ± SD; Range | Females (N=4) Mean ± SD; Range |
|---|---|---|
| SVL | 49.3 ± 1.56; 47.8–51.3 | 57.6 ± 6.83; 47.7–63.3 |
| HL HW | 16.9 ± 0.87; 15.7–17.7 16.2 ± 0.67; 15.4–16.9 | 19.4 ± 2.18; 16.6–21.9 18.4 ± 1.92; 15.6–20.0 |
| EN | 5.0 ± 0.36; 4.5–5.3 | 6.1 ± 0.87; 4.9–7.0 |
| ED TD | 5.9 ± 0.29; 5.1–6.1 3.7 ± 0.22; 3.5–4.0 | 6.1 ± 0.71; 5.1–6.8 4.3 ± 0.70; 3.5–5.2 |
| ELW | 4.8 ± 0.08; 4.7–4.9 | 5.5 ± 0.79; 4.6–6.4 |
| IOD TL | 5.0 ± 0.30; 4.7–5.4 26.5 ± 0.70; 25.7–27.3 | 5.8 ± 0.83; 4.8–6.6 31.9 ± 3.28; 27.2–32.9 |
| FL | 32.5 ± 1.35; 30.5–33.4 | 38.5 ± 4.46; 31.9–41.3 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.