Marthasterias glacialis ( Linnaeus, 1758 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4639.1 |
|
publication LSID |
lsid:zoobank.org:pub:B1690E30-EC81-46D3-881D-97648DDC7745 |
|
DOI |
https://doi.org/10.5281/zenodo.5583214 |
|
persistent identifier |
https://treatment.plazi.org/id/4148D212-0411-FF90-FF33-FD31748B1208 |
|
treatment provided by |
Plazi |
|
scientific name |
Marthasterias glacialis ( Linnaeus, 1758 ) |
| status |
|
Marthasterias glacialis ( Linnaeus, 1758) View in CoL View at ENA
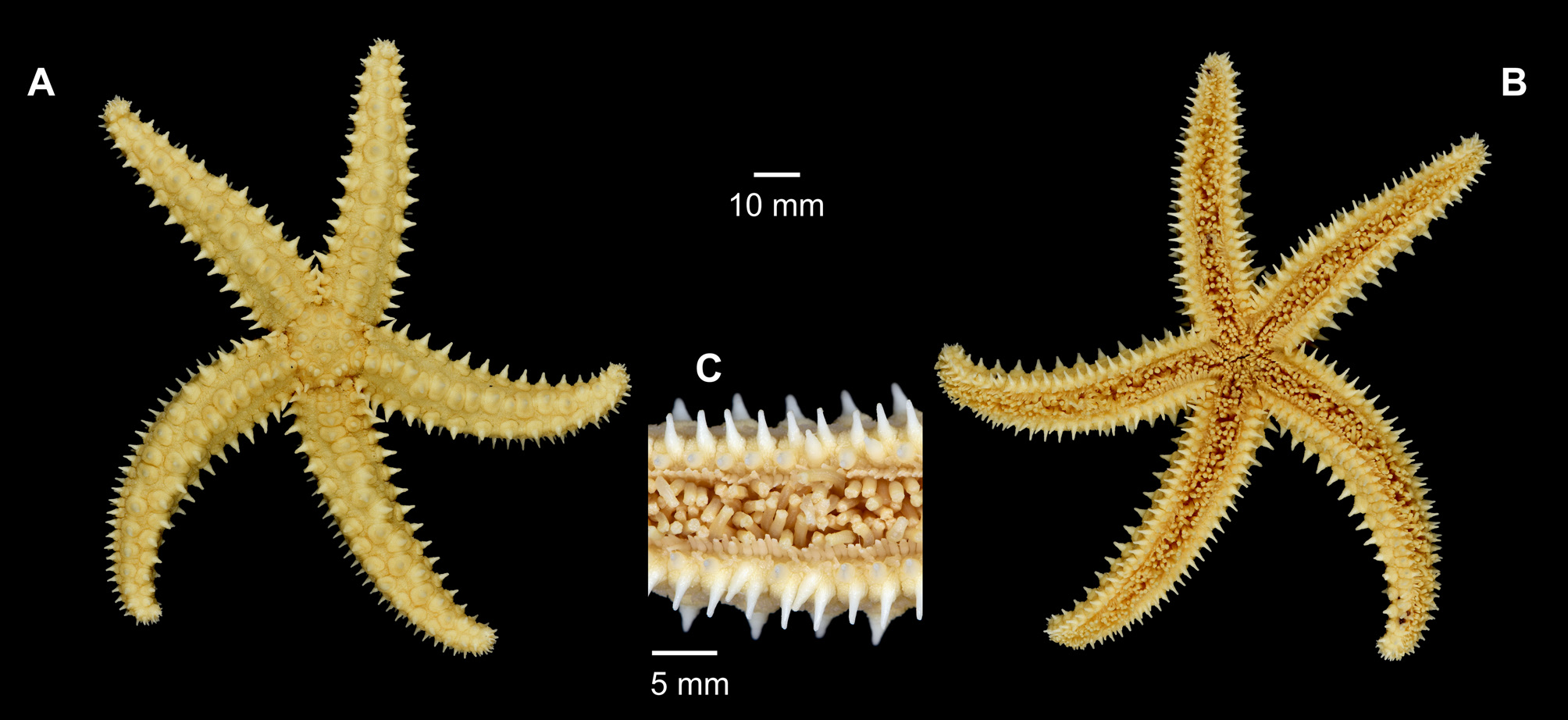
( Fig. 13 View FIGURE 13 )
Reports for the Azores:
Asteracanthion glacialis — $ M̹ller & Troschel 1842: 14–15;
Asterias glacialis Lamarck, 1816 View in CoL —Drouët: 211; Barrois 1888: 31;
Asterias glacialis View in CoL M̹ller, 1776— $ Barrois 1888: 32, 69, 113, 114;
Asterias glacialis Linnaeus, 1758 View in CoL — $ Simroth 1888: 231, 232; $ Koehler 1909: 116; Koehler 1914b: 269; $ Nobre 1924: 88, 1930: 68;
Stolasterias glacialis Linck, 1733 View in CoL — $ Perrier 1894: 109;
Stolasterias madeirensis Stimpson, 1862 — $ Perrier 1896a: 37;
Marthasterias glacialis ( Linnaeus, 1758) View in CoL — $ H.L. Clark 1923: 305; Mortensen 1927a: 143–144, fig. 82; $ Cadenat 1938: 349; Nobre 1938: 34–36, figs. 8, 9; $ Chapman 1955: 400; $ Tortonese 1965: 188–192, figs. 89, 91; $ Marques 1983: 2; Clark & Downey 1992: 443–445, fig. 67d, pl. 101, fig. C; Moyse & Tyler 1995: 671, fig. 12.4; Pereira 1997: 335; $ Morton et al. 1998: 62, figs. 3.4Y, 3.7P, 8.1K; Pérez-Ruzafa et al. 1999: 49, 2002: 281–282; García-Diez et al. 2005: 48; $ Micael et al. 2006: 5, 2010: 329; Micael & Costa 2010: 321; Pérez-Portela et al. 2010: 2015–2028;
Marthasterias glacialis rarispina ( Perrier, 1875) View in CoL — $ A.M. Clark 1951: 211–212;
Marthasterias glacialis View in CoL (M̹ller, 1776)— $ Fisher 1928: 130, pl. 42, fig. 4, pl. 43, fig. 6.
See: Mortensen (1927a); A.M. Clark & Downey (1992); Picton (1993: 36).
Occurrence: Mediterranean Sea and East Atlantic; from Iceland and Finmark ( Mortensen 1927a) along the European and West African coasts ( Nataf & Cherbonnier 1975) to? South Africa (A.M. Clark & Downey 1992), including the archipelagos of the Azores ( Koehler 1909), Madeira ( Augier 1985), Selvagens ( Pérez-Ruzafa et al. 2002), Canaries ( Pérez-Ruzafa et al. 2003) and Cape Verde ( Pérez-Ruzafa et al. 1999).
Depth: 0–180 m, rarely below 50 m (A.M. Clark & Downey 1992); AZO: 0–35 m (herein).
Habitat: found on rocky shores, on biogenic detritus, sandy to silty sand substrates, and in Zostera and Posidonia meadows ( Koukouras et al. 2007); feeds mainly on molluscs but also on fishes, crustaceans and other echinoderms ( Mortensen 1927a).
Larval stage: planktotrophic ( Mortensen1927a);typically gonochoristic, though some cases of hermaphroditism have been reported in the Tyrrhenian Sea ( Delavault & Cognetti 1961).
Material examined: DBUA-ECH 103 (Piscina da Lagoa, SMG, AZO, c. 37°44’29”N, 25°34’27”W, 25.07.199 6, 15 m; 1 spm, R = 83 mm, r = 9 mm); DBUA-ECH 104 (FRM, AZO, c. 37°16’14”N, 24°46’52”W, 1990.06.08, 6–8 m; 1 spm, R = 13 mm, r = 3 mm); DBUA-ECH 105 (Rosto do C„o, S„o Roque, SMG, AZO, c. 37°44’37”N, 25°38’19”W, 1997.02. 26, 10 m; 2 spms, R = 84–101 mm, r = 9); DBUA-ECH 106 (FRM, AZO, c. 37°16’14”N, 24°46’52”W, 1990.06; 2 spms, R = 100–147 mm, r = 12–17 mm); DBUA-ECH 110 (Poços, S„o Vicente, SMG, AZO, c. 37°50’06”N, 25°40’10”W, 1996.04.14; 1 spm, R = 60 mm, r = 8 mm); DBUA-ECH 111 (Vila do Porto, SMA, AZO, c. 36°56’42”N, 25°08’50”W, 1990.06; 2 spms, R = 79–142 mm, r = 11–80 mm); DBUA-ECH 205 (Baixa de Jo„o Lopes, SMA, AZO, c. 37°01’13”N, 25°10’05”W, 2014.06. 26, 30–35 m; 1 spms, R = 1 mm, r = 0.5 mm).
Description: disc subpentagular with scattered spines forming a more or less distinct pentagon; R/ r from two to thirteen in smaller specimens (D = 1–5 mm, respectively) up to 8–11 fold in larger specimens; a larger individual (R = 142 mm, DBUA-ECH 111) was flattened by an inadequate preservation container resulting in a proportion R to r of two fold. Five arms with pentagonal cross-section, long tapering distally. Abactinal skeleton strong, with three longitudinal series of primary plates; the plates in mid-dorsal line of the arm forming a conspicuous regular series, zigzagging distally with one up to two (occasionally three, in larger specimens of R Ξ 78 mm) stout conical spines, encircled by a large wreath of crossed pedicellaria; the dorsolateral series on each side reduced particularly distally, partially spinose with spines (when present) usually smaller than the carinal ones in all specimens except in the smallest ones (R ± 13 mm), where they are absent. Superomarginal plates as in the mid-dorsal arm area arranged in a regular series with one to two spines, slightly exceeding in length the carinal ones; also surrounded by wreath of crossed pedicellaria. Inferomarginal with two oblique spines slightly flattened and of similar length to the abactinal ones; the outer one with crossed pedicellaria surrounding only the outer side. A single spineless actinal series. Adambulacral plates monocanthid. Long lanceolate straight pedicellaria scattered on the ventral surface, particularly within the furrow. Valves of crossed pedicellaria with a slightly enlarged tooth on each side of the terminal lip. Colour (in ethanol): all white, with the exception of a small specimen (DBUA-ECH 104), which is light brown with darker brownish spots giving an overall stripped appearance.
Remarks: Marthasterias glacialis is highly polymorphic and has a wide geographical distribution, qualities that have resulted in the description of several synonyms, subspecies and varieties. The validity of these subspecies did not reunite consensus as many believed to be ecophenotypic variations associated with specific environmental conditions (depth, e.g., Mortensen 1933b; Tortonese, 1965; latitude, e.g., A.M. Clark 1951). Historically, M. glacialis in the Azores was attributed to the form ‘ rarispina ’ by A.M. Clark (1951), a variety originally described as a South African species by Perrier (1875). Its diagnosing characters are essentially focused on the absence of spines on the dorsolateral plate series (e.g., Perrier 1875; H.L. Clark 1923). On the other side of the spectrum is the form ‘ africana ’, another South African variety distinct by its many irregularly arranged abactinal spines ( Mortensen 1933b; A.M. Clark 1974). However, Mortensen (1933b) realized the existence of intermediate forms and classified these as ‘not very distinct varieties’. Unlike the previous observations by A.M. Clark (1951), the specimens from the Azores housed in the DBUA-ECH collection appear to be identical to the ones from South Africa, figured by Mortensen (1933b: pl. 16, fig. 3) as an example of an intermediate form of the variety ‘ rarispina ’ characterized by having a reduced number of spines in the dorsolateral series. In contrast, the carinal plate series in the DBUA- ECH specimens also presented a somewhat zigzagged arrangement in the distal part of the arms. This feature was associated with the typical form ‘ rarispina ’ by A.M. Clark (1951). However, the smallest specimens (R ± 13 mm) in DBUA-ECH collection displayed naked dorsolateral series and the spines on carinal series are not arranged in a zigzagged manner. Regardless, the observed variations found in Azorean animals are not exclusive in the North Atlantic and were also reported in specimens from Madeira, Canaries, Portugal and the Mediterranean Sea ( Ludwig 1897; Nobre 1930; A.M. Clark 1951; A.M. Clark & Downey 1992). The colouration pattern has been appointed as another source of variation in M. glacialis , which can vary between yellow, green, blue, brown and even pink. In the Azores, large specimens are typically described as blackish with the spinose plates conspicuously white (see for example Wirtz & Debellius 2003). However, small specimens (e.g., DBUA-ECH 103, R = 83 mm) may present a colouration pattern similar to typical Coscinasterias tenuispina , light brown to yellowish-white blotched with darker brownish or orange, giving an overall stripped appearance (see remarks under C. tenuispina ). A more recent study by Wright and co-workers (2016) concluded that rarispina and africana varieties from South Africa could not be distinguished at a morphological and genetic level. On the other hand comparisons with sequences previously published by Pérez-Portela et al (2010) from the European shores and Azores showed that South African populations could represent a distinct species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Marthasterias glacialis ( Linnaeus, 1758 )
| Madeira, Patrícia, Kroh, Andreas, Cordeiro, Ricardo, De, António M., Martins, Frias & Ávila, Sérgio P. 2019 |
Marthasterias glacialis rarispina (
| Perrier 1875 |
Stolasterias madeirensis
| Stimpson 1862 |
Asterias glacialis
| Lamarck 1816 |
Asterias glacialis
| Lamarck 1816 |
Asterias glacialis
| Linnaeus 1758 |
Marthasterias glacialis (
| Linnaeus 1758 |
Stolasterias glacialis
| Linck 1733 |