Bacillus amyloliquefaciens, Priest et al., 1987 (ex. Fukumoto, 1943) L. M. Liao, 1987
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2021.112983 |
|
DOI |
https://doi.org/10.5281/zenodo.8239908 |
|
persistent identifier |
https://treatment.plazi.org/id/40239D05-6F76-9B45-FCF7-1CAB8C43C83F |
|
treatment provided by |
Felipe |
|
scientific name |
Bacillus amyloliquefaciens |
| status |
|
2.2. Characterization of the bioactive B. amyloliquefaciens View in CoL View at ENA
The bacterial strain isolated from the rhodophytan marine macroalgae K. alvarezii , with promising antibacterial activity, was identified by inclusive phenotypic and genotypic characterization ( Krieg and Holt 1984; Thilakan et al., 2016), as Gram-positive bacterium with amylase activity. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI- ToF MS) score of 1.92 and 99% similarity in 16S rRNA sequence ( KX272633 View Materials ) coupled with membrane fatty acid composition characterized the bacterial strain as B. amyloliquefaciens , which was deposited as B. amyloliquefaciens MTCC 12713 in the Institute of Microbial Technology (a Budapest Treaty recognized microbial repository of India). Consistent with the preceding reports amongst the order Bacillales , Bacillus sp. were predominantly extant as heterotrophs with a number of marine macroalgae ( Chakraborty et al., 2014), and were documented as promising antibacterial agents ( Li and Vederas, 2009). The Bacillus strains harboring in the distinctive niches of intertidal or deep-water environments, could yield beneficial bioactive metabolites on account of having the innate ability to acclimatize in the extreme surroundings ( Li and Vederas, 2009). Several reports claimed the promising biological activities of polyketide composites produced by marine Bacillus sp. with bioactive pks- gene clusters ( Mondol et al., 2013; Nagao et al., 2001). Therefore, marine heterotrophic Bacillus sp. were endowed with promising antibacterial properties to develop successful management approaches against pathogens of biomedical significance ( Mondol et al., 2011).
2.3. Purification and structural characterization of antibacterial macrobrevin analogues
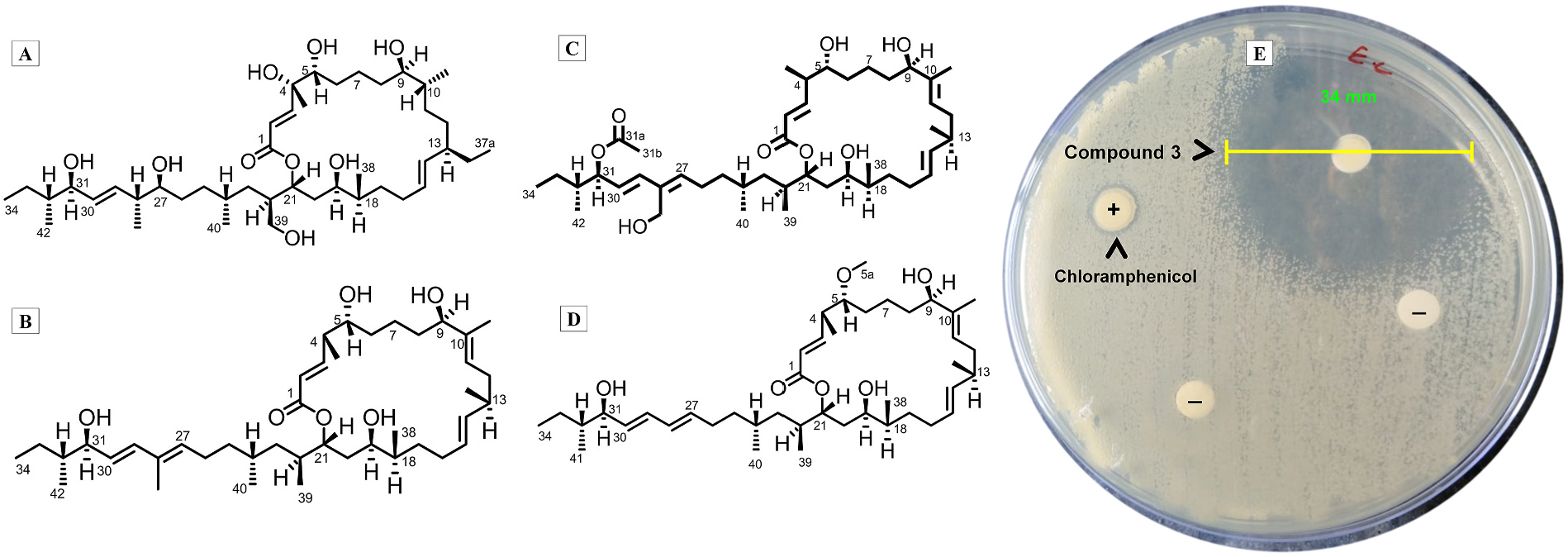
Organic extract of B. amyloliquefaciens MTCC 12713 (~ 5.1 g) was fractionated through flash and sequential preparatory high-pressure liquid chromatographic (HPLC) separation techniques to isolate four homogenous macrobrevin classes of compounds (with ≥98% yield) ( Fig. 1 View Fig ).
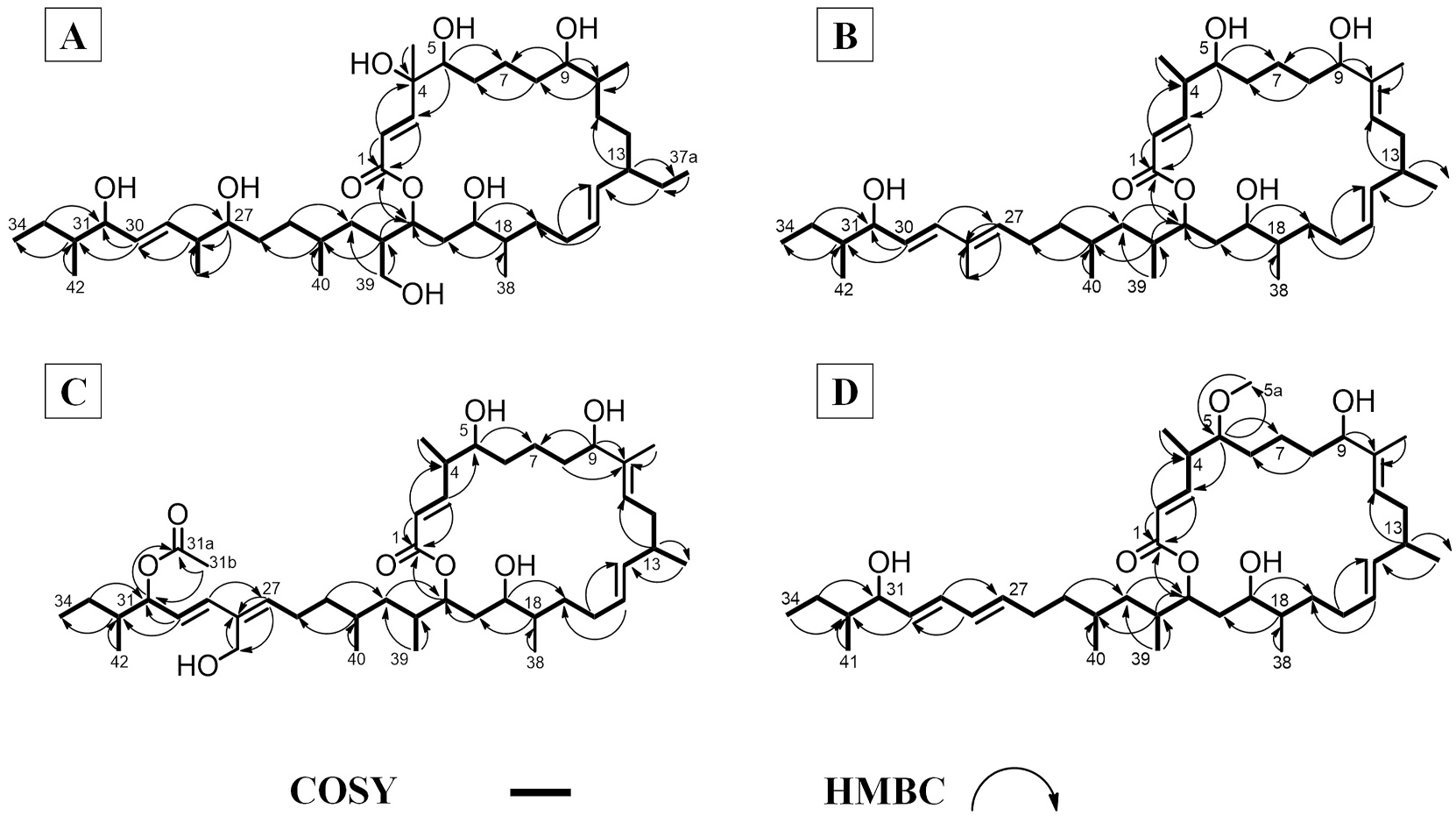
The first in the series of macrobrevin class of compounds was characterized as 4, 27, 39-trihydroxy-decahydro-37-methyl-macrobrevin (compound 1). Its molecular formula was obtained as C 43 H 78 O 9 by mass spectroscopic experiment (HRESIMS m/z 739.5728 [M+H] +), and NMR/IR data ( Table 1A View Table 1 ; Fig. 2A View Fig ; Fig. S1-S View Fig 12). The 13 C/ 135 DEPT NMR represented 43 carbons that included two non-protonated carbons, eight methyls, fourteen methylenes and nineteen methines. Basic framework of macrobrevin skeleton was deduced from spin systems obtained through COSYs at H-2/H-3; H-5/H-14, H-15/H-29 and H-30/H-34, and was further confirmed through the HMBCs. The 21-membered macrocyclic lactone ring was formed between the oxygenated end of ester at C- 1 (δ C 166.2) and methine at C-21 (δ H 3.92/ δ C 70.2), and this was ascribed through HMBCs from δ H 6.05 (H-2), 7.09 (H-3), 3.92 (H-21) to δ C 166.2 (C-1). Previously reported 24-membered macrocyclic lactones exhibited chemical shifts of δ C 167 (C-1), δ H 5.5/ δ C 117–118 (C-2), δ H 6.6/ δ C 145 (C-3), δ H 7.2/ δ C 129–130 (C-4), δ H 6.1/ δ C 140–142 (C-5) and δ H 5.08/ δ H 71 (C-23) ( Gao et al., 2010), which resembled to those recorded in the studied compound 1, as also with another compound, named as bacvalactones showing chemical shifts of δ C 166 (C-1), δ H 5.7/ δ C 121–125 (C-2), δ H 6.1/ δ C 141–143 (C-3) and δ H 4.13/ δ C 72 (C-23) ( Chakraborty et al., 2020). In particular, the proton chemical shift value of δ H 3.92 at H- 21 in the present 21-membered macrocyclic lactone showed change in δ of about ~1–2 ppm as compared to the previously reported 24-membered macrocyclic lactones ( Chakraborty et al., 2020; Gao et al., 2010). This might be due to the absence of conjugated dienes at C-4/C-5 and lesser number of carbon atoms involved in the cyclization of the oxygenated end of ester at C-1 and carboxylated end of methine at C-21. The titled compound was comparable to the previously reported macrobrevin compounds enclosing the alkenes at C-2 = C-3, C-7 = C-8, and C-10 = C-11, C-14 = C-15, C-16 = C-17, C-25 = C-26, C-27 = C-28 and C-29 = C-30 positions ( Helfrich et al., 2018; Soldatou et al., 2019). The alkenic protons with greater coupling constants (J) at δ H 6.05 (J = 13.8 Hz, H-2), δ H 7.09 (J = 12.1 Hz, H-3), δ H 5.48 (J = 13.1, 2.4 Hz, H-14), δ H 5.52 (J = 12.5, 2.1 Hz, H-15), and δ H 5.32 (J = 13.0 Hz, H-29), δ H 5.41 (J = 10.2 Hz, H-30) positions designated their trans- orientation (E). Compound 1 enclosed a hydroxylated quaternary carbon at C-4 (δ C 81.2), which was substantiated through HMBCs from δ H 6.05 (H-2), 3.35 (H-5) to δ C 81.2 (C-4). The dispositions at C-13 and C-22 enclosed ethyl and hydroxymethylene linkages, respectively through HMBCs from δ H 0.93 (H-37a) to δ C 28.1 (C-37) {COSYs δ H 1.41 (H-37)/0.93 (H-37a)} and δ H 3.63 (H-39) to δ C 34.9 (C-22), 38.6 (C-23), respectively. Based upon comprehensive spectral analyses and comparing the similarities and variations with basic core of previously reported macrobrevin analogues ( Helfrich et al., 2018; Soldatou et al., 2019), the compound 1 was characterized as 4, 27, 39-trihydroxy-decahydro-37-methyl-macrobrevin. The compound 1 was not previously known, and found to be the derivative of basic skeleton of already known macrobrevins with functional group variations in their structure, and therefore, it could be considered as previously undisclosed macrobrevin analogue. Relative stereochemical assignments of the protons were assigned by NOESYs and earlier reports ( Helfrich et al., 2018). NOEs from δ H 1.83 (H-32)/2.42 (H-28)/1.69 (H-24)/3.92 (H-21)/1.33 (H-35)/3.35 (H-5)/1.78 (H-10)/0.99 (H-38) were attributed for adjacent spatial apportionments that supported their equivalent plane of alignments, and consequently, assigned as β -oriented. Similarly, NOEs between δ H 3.97 (H-31)/0.88 (H-42)/1.03 (H-41)/0.95 (H-40)/2.21 (H-22)/3.44 (H-19)/1.72 (H-18)/2.15 (H-13)/1.02 (H-36)/3.15 (H-9) were recognized for their adjacent spatial distributions that reinforced their comparable plane of alignments, and accordingly, designated as α -oriented. The mass spectrum exhibited a molecular ion peak at m/z 738, and the base peak was deduced as 2,2-dihydroxyethen-1-ylium cation at m/z 59 (K), which could form by successive fragmentations including McLafferty rearrangements (Fig. S9-10).
Another previously undisclosed macrobrevin analogue polyketidespanned macrolactone (macrobrevin 2) was characterized as 7,8,16,17,25,26-hexahydro-macrobrevin. Its molecular formula was deduced as C 42 H 72 O 6 by mass spectroscopic experiment (HRESIMS m/z found 673.5410 [M+H] +), and combination of NMR/IR data ( Table 1A View Table 1 ; Fig. 2B View Fig ; Fig. S13-S24). 13 C/ 135 DEPT NMR represented 42 carbons, which included three non-protonated carbons, nine methyls, ten methylenes and twenty methines. Similar to the previous compound 1, the 21- membered macrobrevin was deduced by COSYs through H-2/H-9; H-11/H-14, H-15/H-27 and H-29/H-34, and side chain attachment at C-21 was confirmed through HMBC. The titled compound enclosed the alkenic groups only at C-2 = C-3, C-10 = C-11, C-14 = C-15, C-27 = C-28 and C-29 = C-30 positions, when compared to the previously reported macrobrevins ( Helfrich et al., 2018; Soldatou et al., 2019). The alkenes with greater coupling constants at δ H 5.82 (J = 13.1 Hz, H-2), δ H 6.89 (J = 12.1, 4.1 Hz, H-3), δ H 5.19 (J = 10.5 Hz, H-11), δ H 5.46 (J = 13.0, 2.1 Hz, H-14), δ H 5.68 (J = 12.1, 2.5 Hz, H-15), δ H 6.00 (J = 13.9 Hz, H-27), and δ H 6.47 (J = 13.1 Hz, H-29), δ H 5.39 (J = 10.2 Hz, H-30) positions attributed their trans -orientation (E). A deshielded quaternary carbon was present in the previous compound 1 at δ C 81.2 (C-4), whereas compound 2 enclosed a shielded methine carbon at δ H 37.5, which recognized the absence of hydroxyl at this position. Also, a higher chemical shift value of about ~3 ppm at H-27 (δ H 6.00) in the compound 2, when compared to that in 1 (δ H 3.57) suggested the presence of alkenic group in 2, in preference to the hydroxyl in 1 at this location (C-27). Lower chemical shift value of about ~3 ppm at H-39 (δ H 0.94) was observed in 2 when compared to that recorded in 1 (δ H 3.63), and were attributed to the occurrences of hydroxyl in 1 and terminal methyl in 2. NOEs from H-32/H-24/H-21/H-39/H-38/H-37/H-5/H-35 were acknowledged for their β -orientation ( Helfrich et al., 2018), and those between H-9/H-36/H-13/H-18/H-19/H-22/H-40/H-41/H-42/H-31 could recognize their α -positioning. The mass spectrum exhibited a molecular ion peak at m/z 672, and the base peak was realized as 2, 2-dihydroxyethen-1-ylium cation at m/z 59 (J), which was formed by repeated fragmentations including McLafferty rearrangements (Fig. S20-S21).
The molecular formula of previously undisclosed hexahydro 41-hydroxy-macrobrevin-31-acetate (compound 3) was deduced as C 44 H 74 O 8 by mass spectroscopic experiment {HRESIMS m/z found 731.5464 [M+H] +}, and its structure was elucidated by IR/NMR ( Table 1B View Table 1 ; Fig. 2C View Fig ; Fig. S25-S36). 13 C/ 135 DEPT NMR represented 44 carbons, which included four non-protonated carbons, nine methyls, eleven methylenes and twenty methines. The hydroxymethylene at C-28 was substantiated through HMBCs from H-41 to C-28, whereas an acetate linkage at C-31 was set forth through HMBCs from H-31 to C-31a; H-31b to C-31, C-31a. The higher chemical shift value of about ~1 ppm at H-31 (δ H 4.65) in the compound 3, when compared to that in 1 (δ H 3.97), ascribed the presence of highly electronegative oxygenated end of ester (acetate) in 3, whereas 1 enclosed a hydroxyl at this position (C-27). A deshielded non-protonated carbon was present in 3 at δ C 143.8 (C-28), which was bonded to the hydroxymethyl, whereas the compound 1 enclosed a shielded methine at δ H 2.42 (C-28) attached to a terminal methyl group. Thus, the presence of an acetate linkage at C-31 and hydroxymethyl at C- 28 in 3 were assigned, when compared to the presence of hydroxyl at C-31 and methyl at C- 28 in trihydroxy-37- methyl-macrobrevin (1). Similar to the previously discussed compounds 1–2, the titled compound exhibited trans -orientation (E) at their alkenic positions by reason of greater coupling constants. NOE couplings from H-35/H-5/H-37/H-38/H-21/H-24/H-32 and H-21/H-39 were acknowledged for their β -alignment, and those between H-31/H-42/H-22/H-19/H-18/H-13/H-36/H-9 and H-22/H-40 deduced the α -orientation. The mass spectrum exhibited a molecular ion peak at m/z 730, and the base peak was observed at m/z 59 (L) (2,2-dihydroxyethen-1-ylium cation) (Fig. S33-34).
The molecular formula of the previously undisclosed hexahydro-28- nor-methyl-5-methoxy-macrobrevin (macrobrevin 4) was deduced as C 42 H 72 O 6 by mass spectroscopic experiment (HRESIMS: m/z found 673.5410 [M+H] +), and combination of NMR/IR data ( Table 1B View Table 1 ; Fig. 2D View Fig ; Fig. S37-S47). 13 C/ 135 DEPT NMR represented 42 carbons that included two non-protonated carbons, nine methyls, ten methylenes and twenty one methines. The titled compound enclosed methoxy linkage at C-5, whereas its homologue trihydroxy-37-methyl-macrobrevin (compound 1) enclosed a hydroxyl at C-5. A higher chemical shift value of about ~1 ppm at H-5 (δ H 3.35) in compound 1, when compared to that in 4 (δ 2.84) attributed the presence of hydroxyl in 1, whereas 4 enclosed O -methyl end at this place (C-5). Occurrence of methoxy linkage at C-5 was deduced through HMBCs from H-5 to C-5a and H-5a to C-35. The greater chemical shift at H-28 (δ H 6.05) in 4 ascribed the presence of alkene, whereas the previous compound 1 enclosed a methine functionality with shielded proton shift of about δ H 2.42 (H-28), which was attached to a terminal methyl group. The typical macrobrevin enclosed a methyl at C-28 position, whereas the titled compound was devoid of any methyl group at C-28, and therefore, named as normethyl macrobrevin analogue. Alkenic groups in the titled compound were found to be trans -orientated (E) attributable to their greater coupling constants. NOE couplings from H-32/H-24/H-21/H-37/H-5/ H-35 and H-21/H-38 were acknowledged for their β -orientation, and those between H-41/H-31/H-40/H-22/H-19/H-18/H-13/H-36/H-9 could attribute their α -orientation. The mass spectrum exhibited a molecular ion peak at m/z 730, and the base peak was observed at m/z 59 (K, ethene-1, 1-diol cation), which could form through repeated fragmentations through McLafferty rearrangements (Fig. S44-S45).
2.4. Bioactivities of macrobrevin analogues 1–4 purified by bioactivity-
supported fractionation and structure-activity relationship analysis
The column fractions derived by repeated chromatographic separation were evaluated for antibacterial properties against broad spectra of pathogens, and those with higher yield and activities were additionally purified to obtain the pure compounds ( Table S1 View Table 1 ). The fraction (BAM 1-3- 1) with maximum antibacterial activity (~ 26–28 mm zone diameter) was purified to isolate four homogenous macrobrevin classes of compounds.
B. amyloliquefaciens MTCC 12713 demonstrated a wide spectrum of antibacterial activities on spot-over-lawn assay against clinically relevant pathogens (> 20 mm), on preliminary screening. The purified macrobrevins (1 – 4) showed considerable antibacterial activities against the drug-resistant pathogenic strains tested, amongst which compound 3 demonstrated promising inhibitory potential against MRSA (24 mm), VRE fs (34 mm), P. aeruginosa (23 mm) and K. pneumoniae (25 mm) (30 μg on disc) ( Fig. 1 View Fig ; Table 2 View Table 2 ). Notably, small halos do not always mean that MICs are high. Small halos along with low MICs could also occur due to differentiated diffusion of each antimicrobial compound. Vancomycin or teicoplanin are examples of this behavior. In addition, chloramphenicol (30 μg per disc) gave a negligible inhibition zone only against K. pneumoniae ( Table 2 View Table 2 ). Microdilution analysis showed considerable MICs with hexahydro-41-hydroxy-macrobrevin-31-acetate (compound 3) for MRSA, VRE fs, K. pneumoniae and P. aeruginosa (1.56 μg/mL) compared to those displayed by positive controls Footnotes are same as given under Table 1A View Table 1 .
(chloramphenicol and ampicillin with a range of MIC values from 6.25 to 12.5 μg/mL) and 1, 2 and 4 (MRSA/ K. pneumoniae / P. aeruginosa 3.12–6.25 μg/mL, VRE fs 6.25 μg/mL). These results could infer greater antibacterial property of 3 when compared to the commercial standards and other studied compounds. Electronic parameters of titled macrobrevin analogues were found to play a significant role to effect greater bioactive potential. Herein, the electronic parameter, such as polarizability (Pl) of macrobrevin analogues 1–4 were higher (76–84 × 10 24 cm 3) than that of standard antibiotic, chloramphenicol (28.76 × 10 24 cm 3). Also, the electronic parameter as determined by total polar surface area (tPSA) of macrobrevin analogues 1 and 3 were greater (107–167) as compared to those of chloramphenicol (115.38) and other compounds in the series. In particular, higher electronic values of macrobrevin analogue 3 were caused by the presence of greater number of electron-rich centres, including hydroxyls (four in numbers), two ester functionalities and non-conjugated/conjugated dienes. These results demonstrated the correlation among electronegative groups and higher in vitro antibacterial property of 3 compared to other macrobrevin analogues and the standard.
2.5. Core biosynthetic gene cluster associated with macrobrevin analogues and putative biosynthetic pathway
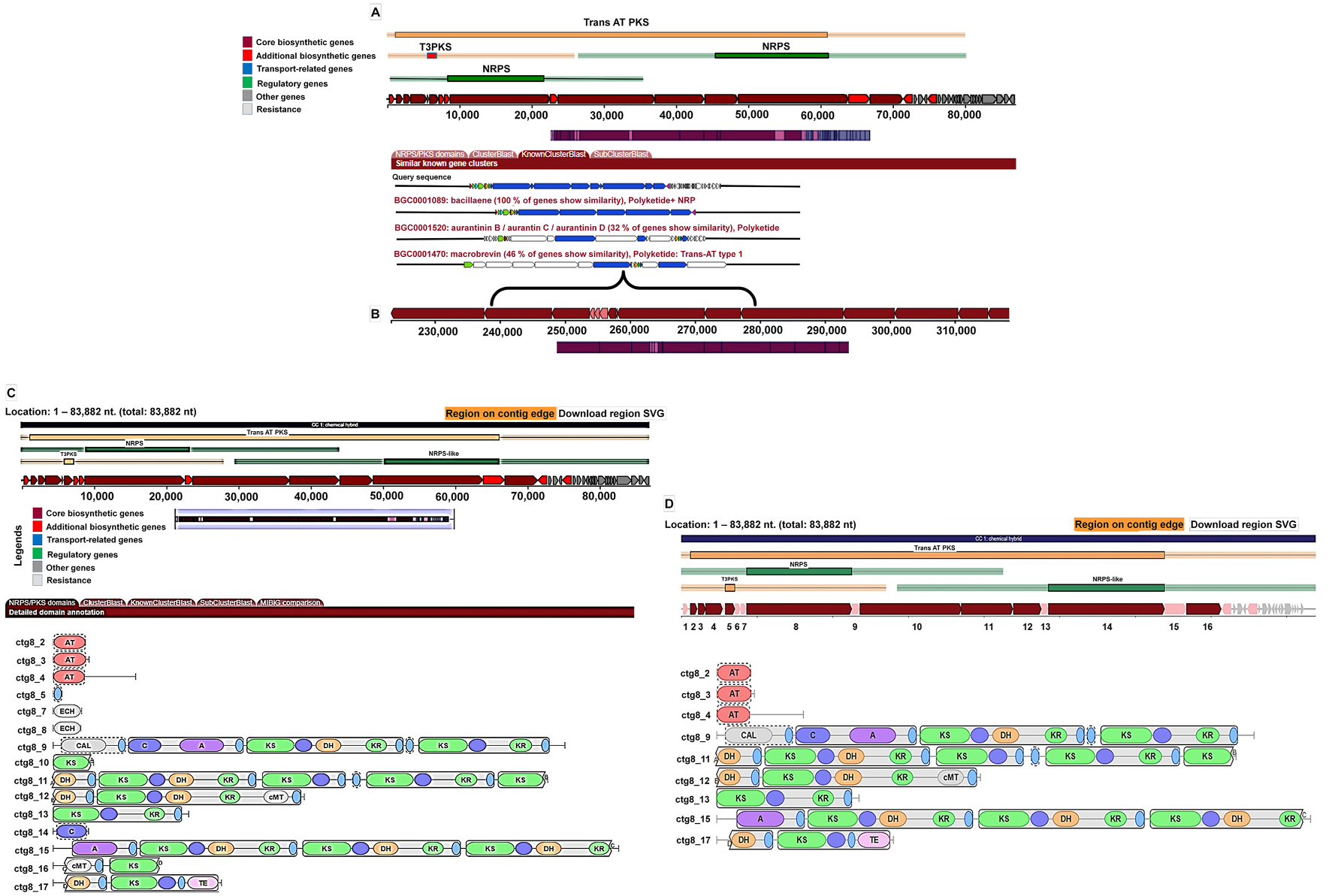
Of late, improvements to characterize the bacterial BGCs have revolutionized the dimensions to comprehend their capacities to produce antibacterial specialized metabolites. Recently, substantial efforts have been directed to relate the genomic information of bacteria to those of the specialized metabolites produced by those ( Soldatou et al., 2019). In the genome mining study, among the 34 biosynthetic gene clusters, a hybrid trans -acyltransferase (trans -AT) pks/nrps gene cluster, which extends up to ~81 Kb, was recognized in the genome of B. amyloliquefaciens MTCC 12713 ( Fig. 3 View Fig ). The pks/nrps gene cluster disclosed 46% similarity to trans -AT PKS-derived macrobrevin isolated from a mesophilic bacterium Brevibacillus sp. Leaf182 associated with the phyllosphere of the wild-type genotype of Arabidopsis thaliana ( Helfrich et al., 2018; Soldatou et al., 2019). Furthermore, the “known-cluster-blast” was utilized to classify prospective products for the genetic clusters from MI-BiG database. Whole genome sequence of the candidate bacterium was submitted in the GenBank with an accession number of QKQQ00000000 (Supplementary material S4). Though the BGC in the genome showed 100% similarity to the bacillaene gene cluster, the metabolic products were turned out to be the derivatives of macrobrevin. Most convincing answer for this switching over of metabolite production is that many specialized metabolite BGCs or individual genes in BGCs are expressed poorly, or not at all, under the laboratory growth conditions owing to their tight control (at the levels of transcription and/or translation) in response to either direct or indirect environmental signals ( Brakhage, 2013). It was also reported that when microorganisms were grown in pure culture in vitro, many of the activating signals were presumably absent, and the expression of specialized metabolite BGCs or any regulatory gene was thus down regulated ( Wezel and McDowall, 2011). It was reported that the elansolids are biosynthesized by trans -AT polyketide synthase of type-1 pks ( Piel, 2010). The proposed functions of genes (1–16) contained in the biosynthetic gene cluster were listed out in Fig. 3 View Fig .
Antibacterial activities were expressed in terms of minimum inhibitory concentration (MIC values were described in parentheses and expressed as μg/mL) and inhibitory zone (expressed in mm) against clinically relevant pathogens.
a-d Row-wise values with different superscripts of this type indicate statistically significant difference (p <0.05). Triplicate values were taken and the variance analyses (ANOVA) were carried out (using Statistical Program for Social Sciences, ver. 13.0) for means of all parameters to examine the significance level (p <0.05). Results were expressed as mean ± SD (n = 3).
The macrobrevin analogues 1–4 and commercial standards were taken 30 μg per disc.
Abbreviations: MRSA = Methicillin resistant S. aureus, VRE fs = Vancomycin resistant E. feacalis .
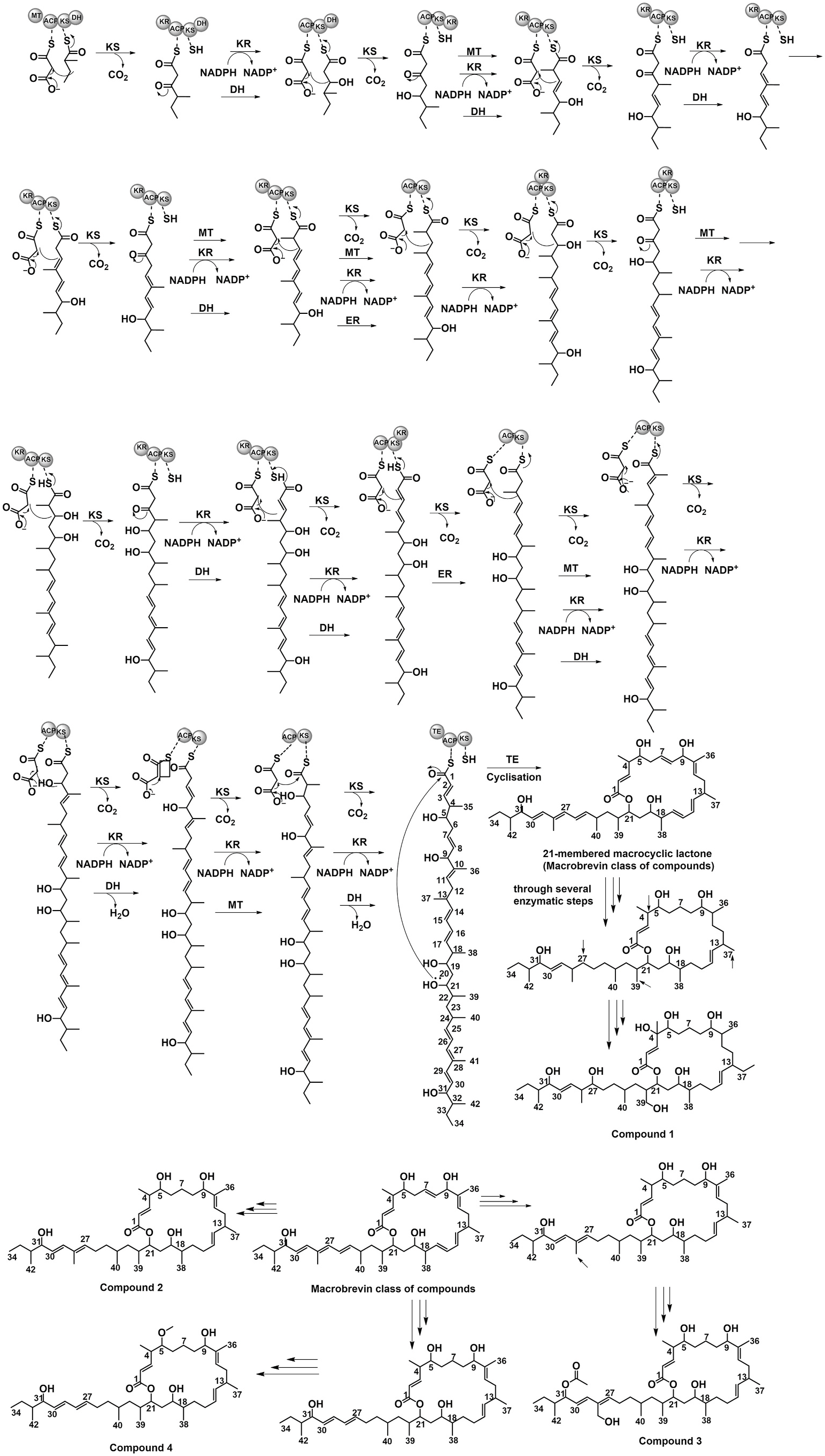
The putative biosynthetic pathway is a proposition based on comparisons with other organisms ( Helfrich et al., 2018; Soldatou et al., 2019). Some of the genes (such as nrps) in the presented biosynthetic gene cluster might not function in macrobrevin biosynthesis as judged from the lack of amino acid moiety in the isolated macrobrevin compounds. At this instant, we have proposed a possible biosynthetic pathway through which the titled compounds 1–4 might be formed. Putative biosynthetic origin of the titled macrobrevin analogues 1–4 was found to be analogous to the biosynthesis of previously described macrobrevins from Brevibacillus sp. ( Helfrich et al., 2018; Soldatou et al., 2019). Herein, the 21-membered macrocyclic lactone part and its extender linear chain at C-21 were found to biosynthesize by the decarboxylative Claisen condensations ( Fig. 4 View Fig ). The biosynthetic route could be originated by condensation of malonate-S-ACP residue with 2-methylbutanethioic-S-KS catalyzed by ketosynthase-mediated decarboxylative Claisen condensation. In the formation of 2-methylbutanoyl-KS initiator group, the KS-assisted decarboxylative condensation was found to be the initial step, followed by methylation at C-2 by methyltransferase/S-adenosyl-methionine and KR-assisted reduction through the conversion of NADPH + to NADP + and DH-assisted dehydration. The elongation steps comprised of 16 modules with ketosynthase, ketoreductase, methyl transferase, dehydratase, enoyl reductase, thioesterase, and acyl carrier protein domains. Repeated additions of malonate groups through Claisen condensations including reduction and methylation could result in the formation of linear chain of 34-membered carbon framework with hydroxyls and methyls in association with C-1 carbonyl carbon. The C-1 carbonyl of the linear chain of 34-membered carbon framework could be cyclized to the nucleophilic hydroxyl at C-21 position through thioesterase-catalyzed biosynthesis leading to the formation of 21-membered macrocyclic lactone (macrobrevin) skeleton.
The double bonds at C-7, C-10, C-16, C-25, and C-27 positions might be saturated through several enzyme-associated biosynthesis. Thereafter, the nucleophilic hydroxyl could be added to the electrophilic position C-27 through enzyme-assisted hydroxylation. The chain elongation at C-13 through various enzyme-assisted mechanism yielded the compound 1. Similarly, the double bonds at C-8, C-16, and C-25 positions could be saturated through the enzyme-assisted biosynthesis to afford compound 2. The latter upon hydroxylation at C-41 and addition of electrophilic acetyl group at the nucleophilic end of hydroxyl at C-31 through several pathways might result in the formation of compound 3. The double bonds at C-8, C-16, and C-25 positions might be saturated and methyl group was eliminated from C-28 position through various enzymatic reactions. Enzyme-assisted methylation at the nucleophilic end of hydroxyl at C-5 might form the compound 4. Although it was predicted that the presented gene cluster could be involved in macrobrevin biosynthesis, more detailed analysis should be necessary for the elucidation of its biosynthetic pathway.
2.6. Molecular docking interaction and drug-likeness
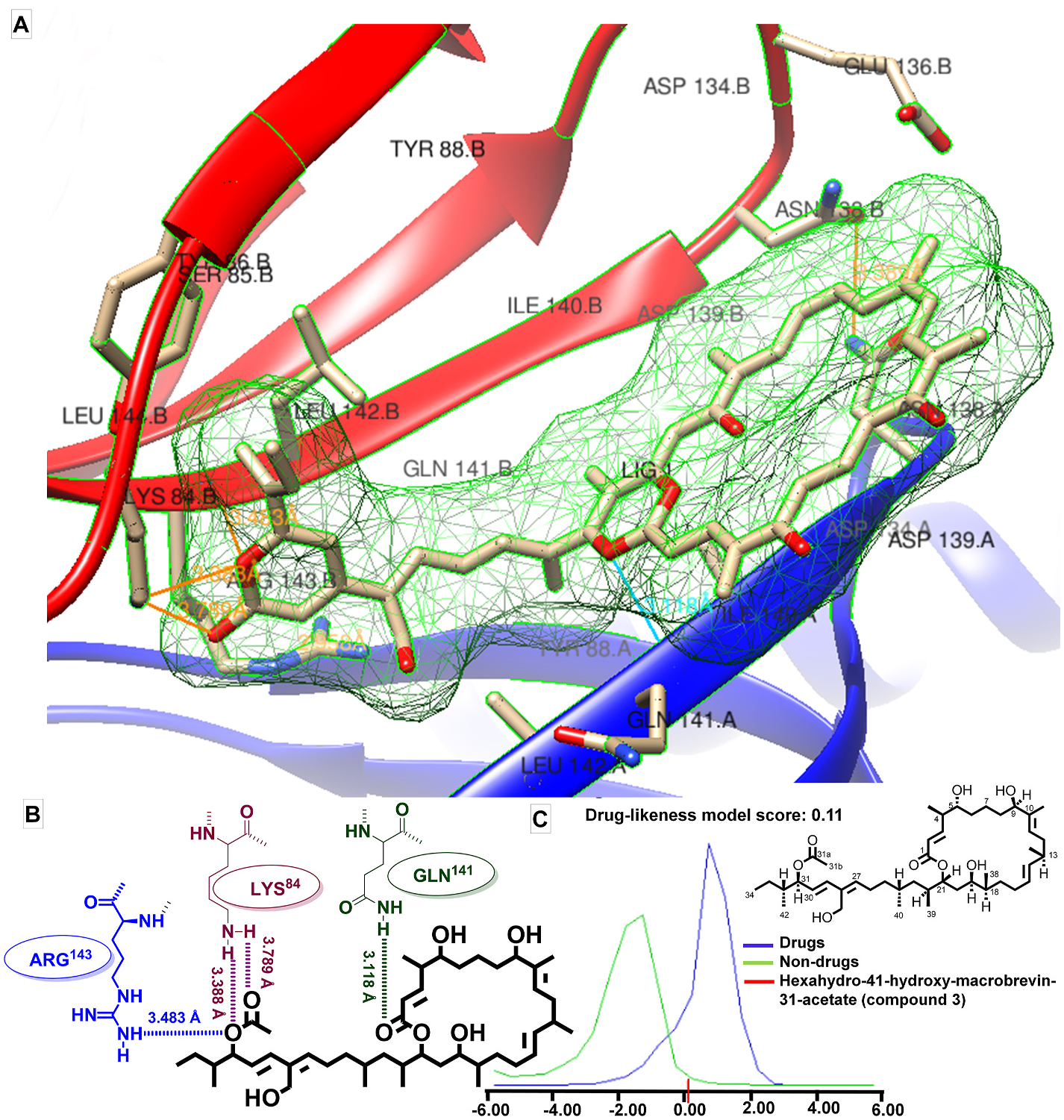
As a means to assign the antibacterial properties of the studied polyketide-bridged macrobrevins (compounds 1-4) from the marine algaassociated B. amyloliquefaciens against peptide deformylase (PDF), the enzyme PDF of S. aureus (1LQW) was docked with the ligands (macrobrevin derivatives). PDF has been labeled as a representative drug target based on the selectivity principles to demonstrate the antibacterial potential against drug-resistant S. aureus , as PDF is critical for bacterial survival. Interestingly, no corresponding functional protein is present in human beings ( Guay, 2007). Additionally, deformylation is a conserved attribute around the eubacteria, and this would permit to characterize wide-ranging antibiotic compounds ( Giglione et al., 2000). It was reported that macrolactin N could inhibit S. aureus peptide deformylase. Considering that the chemical structure of macrobrevin-type compounds is similar to that of macrolactin N, macrobrevin-type compound might also inhibit peptide deformylase. The natural PDIs (peptide deformylase inhibitor), macrolactin N and actinonin were chosen as reference molecules with binding energies of 9.4 kcal/mol and 6.9 kcal/mol, respectively ( Chen and Yuan, 2005). Docking analysis demonstrated the binding energy for compound (3) with PDF of 12.61 kcal/mol coupled with an inhibition constant (K i) of 573.34 pM ( Table 3 View Table 3 ), other than exhibiting maximum number of hydrogen bond interactions among the titled compounds ( Fig. 5 View Fig ). These results demonstrated that hexahydro-41-hydroxy-macrobrevin-31-acetate (compound 3) had greater inhibition potential for PDF ( S. aureus ) than that exhibited by natural PDIs. Further, drug-likeness score showed that the compound 3 disclosed a drug-like quality (drug-likeness score, 0.11). The occurrence of greater number of electron-rich cores, including hydroxyls (four), two ester functionalities, and greater number of non-conjugated/conjugated dienes in macrobrevin analogue 3, when compared to those of other macrobrevin analogues, might contribute to the greater antibacterial properties of the former. The results obtained from the molecular docking/drug-likeness studies affirmed the antibacterial potential of the titled macrobrevin derivatives, more precisely compound 3, as a promising antibacterial lead.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.