Zyzzyzus warreni Calder, 1988
|
publication ID |
https://doi.org/ 10.5281/zenodo.273951 |
|
DOI |
https://doi.org/10.5281/zenodo.6244330 |
|
persistent identifier |
https://treatment.plazi.org/id/3F26D74D-FFCF-8928-FF72-6A09FCF3FE54 |
|
treatment provided by |
Plazi |
|
scientific name |
Zyzzyzus warreni Calder, 1988 |
| status |
|
Zyzzyzus warreni Calder, 1988 View in CoL
Figure 1 View FIGURE 1 , Tables 5–7 View TABLE 5 View TABLE 6 View TABLE 7
Tubularia solitaria Warren, 1906: 83 View in CoL , pls. 10–11, figs. 1–26 (junior primary homonym of Tubularia solitaria Rapp, 1829 View in CoL ).— Warren, 1908: 280.— Millard, 1957: 179; 1966: 434.—de Kruijf, 1977: 19.
Zyzzyzus solitarius ;— Stechow, 1921: 249; 1923: 50.— Kramp, 1949: 198.— Millard & Bouillon, 1974: 2.— Millard, 1975: 40, fig. 16 A–B.— Petersen, 1979: 121.— Wedler & Larson, 1986: 72 –73, fig. 1 A, pl. 1, fig. 3.— Migotto & Silveira, 1987: 104 –106, figs. 4–5.—Hirohito, 1988: 24–26, fig. 7.
Corymorpha solitaria ;— Kramp, 1933: 12.
Zyzzysus solitarius Bandel & Wedler, 1987: 39 [incorrect subsequent spelling].
Zyzzyzus warreni Calder, 1988: 49 View in CoL –50, figs. 38–40; 1991: 2068.— Migotto, 1996: 25.
Zyzzyzus calderi Petersen, 1990: 180 View in CoL –181: fig. 30 A–C.— Calder, 1993: 66; 1998: 1850.—Marques & Migotto, 2001: 469 [new synonymy].
Material examined. Preserved: South Africa: Bat’s Cave Rocks, East London (33°01’S, 27°54’E), 30 Dec 1948, three hydroids without gonophores, embedded in sponge, SAM H–3589; several hydroids with gonophores in sponge, SAM H–2648 cp 326 D; Langebaan Lagoon (33°09’S, 18°03.4’E), 15 Jul 1946, several hydroids with gonophores, embedded in sponge, SAM H–2649; Oudekraal (33°58.5’S, 18°22.2’E), 15 Mar 1934, several hydroids without gonophores, embedded in sponge, SAM H–2647; Oudekraal (33°58.5’S, 18°22.2’E), 15 Mar 1934, microslide with five hydroids without gonophores, SAM H–2647 A122; Cape Peninsula (ca. 34°10’S, 18°30’E), microslide with three hydroids without gonophores, SAM H–2648 cp 326 D; St. James False Bay (ca. 34°10’S, 18°30’E), 1912, coll. and det. K.H. Barnard, several hydroids with gonophores, embedded in sponge, SAM H–398. Cape Verde Archipelago: São Vicente (ca. 16°54’N, 25°00’W), 30 Jul 1904, microslide with detached gonophores, coll. and det. J. Ritchie, NMS 1959.33.39. Bermuda: Flatt’s Inlet (ca. 32°19’N, 64°44’30”W), 1-2 m, 13 Sep 1977, several hydroids up to 5 mm high, some with developing blastostyles, embedded on Tedania ignis , coll. and det. D. Calder, ROMIZ B 133; Flatt’s Inlet (ca. 32°19’N, 64°44’30”W), 0 2 Aug 1982, 3 m, on underside of flat rock, several hydroids up to 10 mm high, with developing blastostyles, embedded in sponge and Eudendrium carneum , coll. and det. D. Calder, ROMIZ B 147; Castle Grotto, Castle Harbour (ca. 32°21’N, 64°42’W), 20 Jul 1982, 1 m, 25 m inside cave entrance, several hydroids up to 11 mm high, with developing blastostyles, embedded in sponge, coll. and det. D. Calder, ROMIZ B 165. Brazil: São Paulo state, São Sebastião, Ponta do Jarobá, (ca. 20°45’S, 45°26’W), 2 m, 0 3 May 1985, coll. A.E. Migotto, several hydroids without gonophores, embedded on Mycale laxissima , MZUSP 1987; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 1.5m, 26 Mar 2002, on Mycale angulosa , coll. C. Campos, MZUSP 2000, 1998 and 1996; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 13 May 2001, on Mycale angulosa , coll. C. Campos, MZUSP 1993; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 27 May 2001, on Mycale angulosa and Hypnea cervicornis , coll. O.M. Oliveira, MZUSP 1991; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 1m, 26 Mar 2002, on Mycale angulosa , coll. C. Campos, MZUSP 1989; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 11 Jan 1988, on Mycale laxissima , , coll. A. E. Migotto, MZUSP 1985; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 4.5m, 24 Jan 2002, coll. C. Campos, MZUSP 1982; Laje dos Moleques (ca. 23°49’72”S, 45°24’78”W), 4 m, 14 May 1987, several hydroids without gonophores, on Mycale laxissima , coll. A.E. Migotto, MZUSP 1986; São Sebastião, test panel at Ponta do Jarobá, (23°49’42”S, 45°25’16”W), 18 Feb 1992, several hydroids without gonophores, on Hypnea cervicornis and Eudendrium sp., coll. A.E. Migotto, MZUSP 1988; São Sebastião, Ponta do Jarobá, (23°45’S, 45°26’W), 1m, 24 Jan 2002, in sponge, coll. C. Campos, MZUSP 1999; São Sebastião, test panel at Ponta do Jarobá, 0 6 Oct 1989, several polyps with gonophores, on Clavelina oblonga , coll. A.E. Migotto, MZUSP 1995; São Sebastião, test panel at Ponta do Jarobá, (23°49’42”S, 45°25’16”W), 2 m, 13 May 1991, several hydroids with gonophores, embedded on Mycale angulosa , coll. A.E. Migotto, MZUSP 1994; São Sebastião, test panel at Ponta do Jarobá, (23°49’42”S, 45°25’16”W), 15 Jan 1985, several hydroids without gonophores, embedded on Mycale angulosa , coll. A.E. Migotto, MZUSP 1981; Ponta do Jarobá (ca. 20°45’S, 45°26’W), 27 Nov 1984, 2.5m, several detached hydroids with gonophores, coll. F.L. da Silveira, MZUSP 1992; Ponta do Baleeiro (ca. 23°49’45``S, 45°25’24”W), 27 Aug 1985, 0.5m, several hydroids with gonophores, on Zoanthus sp., coll. A.E. Migotto, MZUSP 1990; Ponta do Jarobá (ca. 20°45’S, 45°26’W), 27 Nov 1984, 2.5m, several hydroids with gonophores, embedded on Mycale angulosa , coll. F.L. da Silveira, MZUSP 001984;
Live. São Sebastião, test panel at Ponta do Jarobá, (23°49’42”S, 45°25’16”W), 13 Oct 2001, several hydroids with gonophores, coll. C. Campos, MZUSP 1983; São Sebastião, test panel at Ponta do Jarobá, (23°49’42”S, 45°25’16”W), 25 Jul 2001, several hydroids without gonophores, on Hypnea cervicornis , coll. A.E. Migotto, MZUSP 1980; São Sebastião, test panel at Ponta do Jarobá, (23°49’42”S, 45°25’16”W), 23 Jan 2002, 1.5m, several hydroids with gonophores, on colonial ascidians, coll. A.E. Migotto, MZUSP 1997.
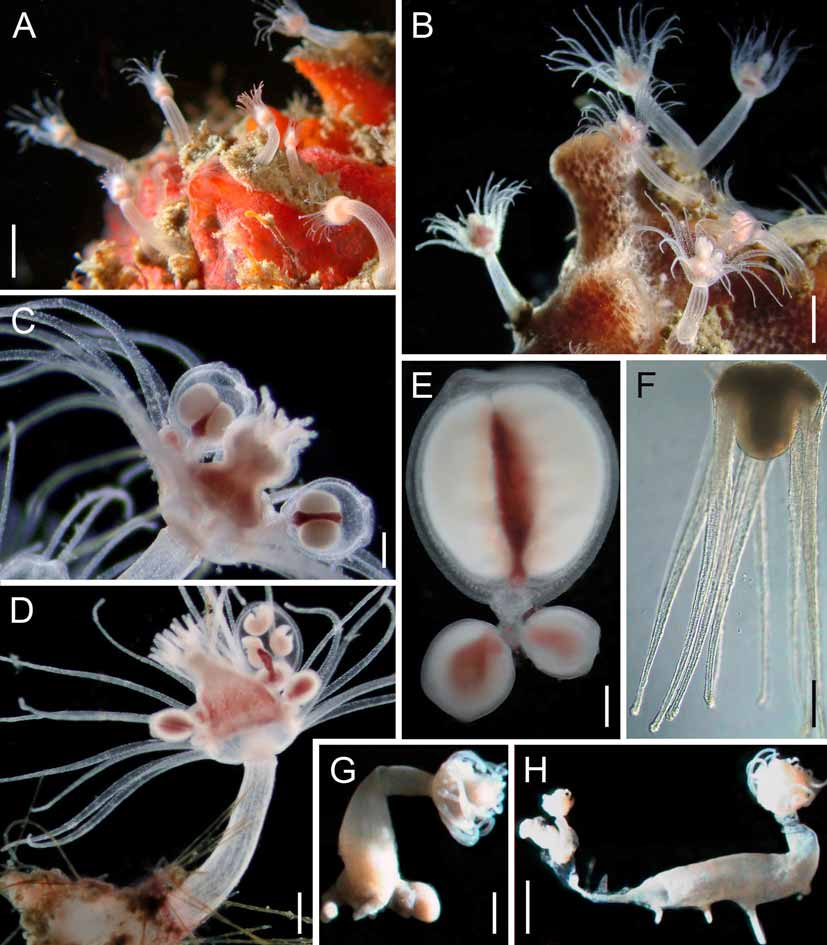
Description. Hydroids 2.8–12.1 mm in height, extent of hydrocaulus embedded in sponges varied, also on tunicates, algae, and other cnidarians. Hydrorhiza with one to several bulbous, finger-shaped or clubshaped processes, 1.8–4.4 mm in length, 0.4–1.4 mm in diameter. Hydrorhizal processes grouped together at most basal part of hydrorhiza or spread around median parts of hydrocaulus, forming anchoring processes. Hydranth vasiform, hydranth body 0.4–1.8 mm high, 0.2–1.0 mm in maximum diameter. Oral tentacles filiform, 7–18 in number, in one whorl, adnate to hypostome around mouth, tentacles circular in cross section, 0.17–0.62 mm long, 0.02–0.12 mm in maximum diameter. Aboral tentacles filiform, 16–24 in number, in one whorl, evenly spaced at base of hydranth body, space between adjacent tentacular bases equal to diameter of tentacles, tentacles ovate in cross section near base, circular in cross section at distal free part, 0.4–2.0 mm long, 0.03–0.15 mm in maximum diameter. Fertile hydranths with 3–10 short, dichotomously ramified blastostyles, in one whorl above aboral tentacles. Hydranths monoecious, each blastostyle with 1–8 cryptomedusoid gonophores, male and female gonophores on different blastostyles; gonophores oval, thickened distal end forming vault around orifice, 4 short, laterally compressed crests at distalmost end. Developed gonophores with large central spadix; male gonophores 0.14–0.72 mm long, 0.14–0.38 mm in maximum diameter; female gonophores 0.7–0.9 mm long, 0.5–0.9 mm in maximum diameter. Completely developed female gonophores with up to 4 actinulae. Actinulae 0.40 mm in height, 0.45 mm in maximum diameter, with 6–16 slightly capitate aboral tentacles. Hydrocaulus flexible, sometimes twisted or bent in several directions, covered by thin perisarc, slightly thickened at basal part of hydrocaulus; hydrocaulus widening proximally, distal part of hydrocaulus cylindrical. Some hydroids growing horizontally on algae with median part of hydrocaulus widened. Perisarc secreted at region above circular groove, between hydrocaulus and hydranth base. Coenosarc of hydrocaulus with 7–10 longitudinal canals, 0.023–0.091 mm in diameter, uniformly aligned at most distal part, irregularly anastomosed along hydrocaulus length. Living hydroids with hydrocaulus well demarcated from hydrorhiza by perisarc thickness, near basal embedding region.
Cnidome. Large stenoteles 9.2–13.7 x 8.2–13.2 Μm (11.5 ± 1.1 x 10.6 ± 1.3, n = 51); small stenoteles 5.1– 7.1 x 3.9–5.9 Μm (5.9 ± 0.4 x 4.8 ± 0.5, n = 100); basitrichous isorhizae 5.7–7.6 x 1.8–3.2 Μm (6.7 ± 0.4 x 2.3 ± 0.2, n = 55); heterotrichous microbasic euryteles 8.0–11.8 x 3.7–6.7 Μm (9.8 ± 1.2 x 5.3 ± 0.9, n = 11); desmonemes 3.4–4.7 x 2.4–3.6 Μm (4.0 ± 0.3 x 3.0 ± 0.3, n = 45). Homotrichous microbasic euryteles 6.8–8.4 x 4.9–6.5 Μm (7.6 ± 0.4 x 5.5 ± 0.4, n = 26) (hydranth); merotrichous isorhizae 6.8–8.0 x 3.4–4.4 Μm (7.6 ± 0.3 x 3.9 ± 0.3, n = 35) (only seen on gonophores) (see Table 6 View TABLE 6 for detailed distribution of basitrichous isorhizae and desmonemes).
Additional data. Warren (1906) described the development of a new hydranth from an adult hydrocaulus. Such regenerative potential under adverse conditions would be associated with additional nutrients stored in the hydrorhiza. Migotto & Silveira (1987: 106, fig. 4) also observed and figured the “budding of a new trophosome starting from a hydrocaulus of a well developed trophosome” (original text in Portuguese). We believe that these observations are correct because occasional growth of some new hydroids, budding off basally in the same hydrocaulus, were also observed by us. However, it is not clear if this is a result of aggregated settlement of actinulae or some kind of regenerative process (or asexual reproduction) of the polyps.
Colour. Tentacles, hypostome, and hydrocaulus translucent to white near most proximal part; hydranth base bright orange; cryptomedusoids milky white with reddish spadix.
Type. South Africa: St. James False Bay (ca. 34°10’S, 18°30’E), 1912, several hydroids without gonophores, embedded in sponge; hydrocauli 1.2–4.2 mm in length; hydranth 0.8–1.7 mm in length, 0.4–1.0 mm in maximum diameter; aboral tentacles 1.2–2.0 mm in length, 0.1–0.2 mm in maximum diameter, coll. K.H. Barnard, SAM H–398.
Additional material assigned to Z. warreni [not studied by us]. Japan: Sagami Bay, hydroids with gonophores, Hydr. 3304–3329 (Hirohito 1988). Brazil: Pernambuco state, hydroids collected along a mangrove swamp on the rivers Porto Alegre and Ariquindá, deposited at ROM ( Calder & Maÿal 1998). Puerto Rico: La Parguera, 1 m, hydroids with gonophores, on Tedania sp. and on Rhizophora sp. roots, USNM 60726, ( Wedler & Larson 1986). South Africa: Oudekraal (33º58.5’S, 18º22.2’E), 15 Mar 1934, west coast of Cape Peninsula, SAM A–122; west coast of Langebaan Lagoon (33º09.0’S, 18º03.4’E), 15 Jul 1946, 7 m, coll. Day, 1959, SAM LB –166; west coast of Saldanha Bay (33º02.5’S, 18º02’E), Sep 1957, SAM SB –153 U; south coast of East London (33º01’S, 27º54’E), Jul 1937, SAM L–172 ( Millard 1966).
Remarks. The diagnostic characters for this species are the two different classes of homotrichous microbasic euryteles, the presence of merotrichous isorhizae (though this character is uncertain for Z. floridanus because of the scarcity of fertile material); male and female gonophores present on different blastostyles (again inconclusive for Z. floridanus because of the scarcity of fertile material), and the distal end of the gonophore with four crests.
The cnidome as described herein differs from previous accounts by Calder (1988), Migotto & Silveira (1987), and Millard (1975), who did not report heterotrichous microbasic euryteles, homotrichous microbasic euryteles [probably referred by Calder (1988) as “?mastigophore”] and merotrichous isorhizae [probably referred by Calder (1988) as “ovate isorhiza”]. Access to large quantities of polyps during this study likely favoured our conclusions.
Our observations on Z. warreni corroborate most of previous reports regarding a single whorl of oral tentacles, morphology of oral and aboral tentacles, place and structure of perisarc secretion, number of blastostyles, and morphology of gonophores. However, some important differences were also noted, such as number of tentacles, internal and external morphology of the hydrocaulus, structure of blastostyles, and nematocyst composition. We discuss these differences below.
Reports on the numbers of oral and aboral tentacles have been quite variable: 16–21 oral, 16–21 aboral ( Warren 1906); 16–21 oral, 15–34 aboral ( Millard 1975); 16–20 oral, 18–25 aboral ( Wedler & Larson 1986); 12 oral and 24 aboral (Hirohito 1988); 15–20 oral, 22–25 aboral ( Calder 1988); 15–21 oral, 15–34 aboral ( Petersen 1990). However, hydranths of Z. warreni may develop new tentacles during ontogeny (pers. obs.), and such differences likely reflect tentacle counts that were made on different developmental stages of preserved materials. This also explains the different morphometric values given in Migotto & Silveira (1987).
Petersen (1990) described Z. calderi based on part of the material ROMIZ B370, previously described as Z. warreni by Calder (1988) from the Bermuda. Petersen (1990: 181) justified the new species based on a series of characters, viz. “the extreme relative length of the neck region, the lower number of endodermal canals, the shape of the hydrocaulus, and the hydrorhiza which in Z. calderi consists of two to three small, club-shaped tubers with finger-like distal processes; the hydrorhiza in Z. warreni consists of large, flesh storage tubers and thin supporting rootlets. Desmonemes in Z. calderi measure 3.7 x 2.8–4.2 x 2.9 μm, in Z. warreni 4.5 x 3.6–6.3 x 3.6 μm, and the isorhizas in Z. calderi measure 6.4 x 1.9–7.3 x 2.1 μm, those of Z. warreni measure 8.1 x 3.6–9.0 x 3.6 μm.” However, as seen in Table 7 View TABLE 7 , the nematocysts of the two species have different proportions.
Although Petersen did not provide morphometric values to document the “extreme relative length of the neck region”, he described the “neck region [of Z. calderi ] long and slim, four to five times as long as hydrocaulus proper” ( Petersen 1990: 180). Meanwhile, Petersen (1990: 182) described Z. warreni with “neck region nearly as long as hydrocaulus proper”. However, we maintain that there is no clear morphological differentiation between distal and median regions of the hydrocaulus (see discussion of Z. floridanus above).
The existence of “about 16 longitudinal canals of equal size” ( Petersen 1990: 182, for Z. warreni ) is not corroborated by our observations, although the reported number is quite varied (“about 16” for Millard 1975; “about ten” for Hirohito 1985). We counted numbers of canals in living polyps and measured histological cross sections at proximal, median, and distal parts of the hydrocaulus. Our results clearly show that canals are open distally, totally closed proximally, and have several anastomoses along the hydrocaulus; moreover, some canals are wider than others and are present in varied numbers (7–10). Combining our data with literature reports, we conclude that the number of canals varies, from 7 (in Brazilian specimens) to 16 (in South African specimens). The proposed diagnostic feature of Z. calderi falls within this range.
The supposed differences in dimensions of desmoneme and basitrichous isorhiza nematocysts are not upheld in a broader, non-regional analysis. Pooling data from various regions ( South Africa, Bermuda, and Brazil, see Table 5 View TABLE 5 ), the “gap” between the putative species disappeared and the conclusion of Petersen (1990) of differences between them is no longer valid.
Petersen (1990: 181) described the hydrorhiza of Z. calderi as “consisting of one to three short, finger- or club-shaped tubers which may split up into finger shaped processes” and that of Z. warreni as “hydrorhiza developed as slender rootlets and swollen tubers of varying size and shape” ( Petersen 1990: 182). Warren (1906: 85) was the first to describe “branches of two kinds: thin rootlets…and thickened fleshy structures”, also observed by Hirohito (1988: 24) (“tuberous root of sweet potatoes in shape, branched or not branched, usually producing several slender processes”). We believe that the morphology of the hydrorhiza should be adopted carefully as a diagnostic character, as it is variable and dependent on the substratum type or host structure. We are convinced that this character is not reliable in describing new species within this genus.
Conspicuous reproductive characters that might differentiate Z. warreni from Z. calderi were not listed by Petersen (1990). He (page 181) described the gonophores of Z. calderi as follows: “gonophores developed […] male and females on same blastostyle” and those of Z. warreni as: “male and female gonophores produced by same hydroid on separate blastostyles” ( Petersen 1990: 182). However, Calder (1988: 50) described the same materials as “in examined specimens, gonophores incompletely developed”, as represented in his figure 39. Although we could confirm the information for Z. warreni , we did not check the only specimens assigned to Z. calderi . Attempts were made by D.R. Calder to locate the type material of Z. calderi at the ROM, without success. For these reasons, we regard Z. calderi as a junior synonym of Z. warreni . More studies on material from other regions is needed to enhance the knowledge of this genus and species.
Distribution. Northwestern Atlantic: Puerto Rico, La Parguera (17°50’N, 67°02’W) ( Wedler & Larson 1986). Bermuda, Castle Grotto, Castle Harbour (ca. 32°21’N, 64°42’W); Flatt’s Inlet (ca. 32°19’N, 64°44’30”W) ( Calder 1988, 1993); Harrington Sound (ca. 32°20’N, 64°44’W). Belize, Twin Cays (ca. 17°29’N, 88°10’W) ( Calder 1991). Netherlands Antilles, Curaçao (ca. 12°10’N, 69°W) (de Kruijf 1977). Colombia, Santa Marta (ca. 11°18’N, 74°10’W) ( Bandel & Wedler 1987). Eastern Atlantic: (?) Cape Verde Archipelago, São Vicente (ca. 16°54’N, 25°W) ( Rees & Thursfield 1965, Watson 1978, Wedler & Larson 1986). Nigeria, Bonny Town jetty, eastern Niger delta (ca. 4°25’N, 7°10’E) ( Petersen 1990). Southwest Atlantic: Brazil, São Paulo state, São Sebastião Channel (23°49’42”S, 45°25’16”W) ( Migotto & Silveira 1987, Migotto 1996); Pernambuco state, Porto Alegre and Ariquindá delta rivers (ca. 08°43’20”S, 35°06’20”W) ( Calder & Maÿal 1998). Southwest Atlantic: off South Africa, False Bay (ca. 34°10’S, 18°30’E); Strandfontein (ca. 34°10’S, 18°50’E) ( Millard 1957); Cape Peninsula, Oudekraal (33°58,5’S, 18°22,2’E), Langebaan Lagoon (33°09’S, 18°03,4’E), Saldanha Bay (33°02.5’S, 18°02’E) ( Millard 1966). Western Indian: off South Africa to Mozambique (21°S, 35°E; 25°S, 32ºE; 29°S, 31°E; 30°S, 30°E; 33°S, 27°E; 34°S, 18°E; 33°S, 18°E) ( Millard 1975); East London (33°01’S, 27°54’E) ( Millard 1975); Tongaat, Isipingo, Park Rynie (30ºS, 30°58’E) ( Warren 1908). Mozambique, Inhaca Island, Cabo da Inhaca (25°58’S, 32°59’E), Santa Carolina Island (21°37’S, 35°20’E) ( Millard & Bouillon 1974). Northwest Pacific: Japan, Sagami Bay (ca. 35°N, 139°30’E) (Hirohito 1988).
Ecology. Substratum.— Z. warreni has been described for many years as living in exclusive association with sponges ( Millard 1975, Migotto & Silveira 1987). In his original description, Warren (1906: 83) observed Z. warreni “on a certain siliceous dark maroon coloured sponge and on a siliceous sponge”. Calder (1988: 50) had observed polyps “usually, but not exclusively, epizoic in sponges […] few specimens were found attached to the hydrocaulus of the hydroid Eudendrium carneum ” from Bermuda, and subsequent authors ( Migotto 1996, Marques & Migotto 2001) also considered the association between Z. warreni and sponges as not obligatory. We have made experiments with actinula settlement (data to be published elsewhere) and found that substratum may also influence on the morphology of the hydrorhizal anchoring processes. This observation has implications for the taxonomy of the genus. Polyps living on algae develop anchoring processes arising at median parts of the hydrocaulus, while polyps living on other cnidarians or sponges develop mucous adherent substances and morphologically simple hydrorhizal processes.
Seasonality. Calder (1988) described a period of dormancy in Z. warreni during winter in Bermuda. In Brazil, some species occurring at São Sebastião Channel also become dormant ( Marques 1993, Migotto 1993). Seasonal studies show that larval recruitment and initial development in Z. warreni occurs in less than 5% of the year (Migotto et al. 2001), and dormancy is a good explanation for its presence. Our observations showed the presence of the species from October to May, occasionally in August, in the São Sebastião Channel.
Depth. Zyzzyzus warreni is a coastal infra-littoral shallow-water species, never observed below 10 m depth.
Associations. Polychaetes of the family Spionidae , small anemones, and ophiuroids actively feed on actinulae and polyps of Z. warreni . Guerrazzi (1999) observed that Z. warreni polyps are a small proportion of the diet of a starfish, Echinaster brasiliensis , which also feeds in the sponges M. angulosa and T. ignis , common substrata of Z. warreni in the São Sebastião Channel. The hydroid is frequently colonized by the ciliate Licnophora sp., which seems to be immune to the nematocysts.
TABLE 6. Comparison of dimensions of nematocysts of Zyzzyzus warreni from Bermuda, Brazil, and South Africa. All data from this study.
| Nº of Length (in Μm) polyps Range x ± SD | n | Width (in Μm) Range x ± SD n | Range of Abundance length:width ratio |
|---|---|---|---|
| Brazil (live material) | |||
| Basitrichous isorhizae Oral tentacles 4 5.8–7.1 6.3 ± 0.3 | 28 | 1.7–2.9 2.1 ± 0.3 28 | 2.45–3.43 Few |
| Aboral tentacles 6 6.1–7.5 6.6 ± 0.3 | 73 | 1.8–2.8 2.2 ± 0.2 73 | 2.66–3.42 Few |
| Hydranth 1 5.7–7.6 6.7 ± 0.4 Hydrocaulus 3 6.1–7.3 6.6 ± 0.3 | 55 26 | 1.8–3.2 2.3 ± 0.2 55 1.5–2.5 2.1 ± 0.2 26 | 2.42–3.10 Numerous 2.86–3.98 Few |
| Desmonemes | |||
| Oral tentacles - - - Aboral tentacles 6 3.5–4.5 4.0 ± 0.2 | - 100 | - - - 2.5–3.8 3.1 ± 0.2 100 | - - 1.21–1.37 Numerous |
| Hydranth 1 3.4–4.7 4.0 ± 0.3 | 45 | 2.4–3.6 3.0 ± 0.3 45 | 1.29–1.41 Numerous |
| Hydrocaulus 3 3.3–4.3 3.8 ± 0.2 South Africa (SAM H–2648) | 45 | 2.2–3.3 2.9 ± 0.2 45 | 1.29–1.49 Numerous |
| Basitrichous isorhizae | |||
| Oral tentacles 1 6.3–6.8 6.6 ± 0.2 Aboral tentacles 1 6.1–7.0 6.7 ± 0.2 | 8 28 | 2.0–2.5 2.3 ± 0.1 8 2.0–3.4 2.3 ± 0.3 28 | 2.73–3.12 Numerous 2.05–3.12 Numerous |
| Hydranth 1 5.7–7.6 6.7 ± 0.5 | 22 | 1.8–3.5 2.4 ± 0.4 22 | 2.19–3.09 Numerous |
| Hydrocaulus 1 6.3–6.7 6.5 ± 0.3 Desmonemes | 2 | 2.1–2.2 2.1 ± 0.1 2 | 3.02–3.09 Numerous |
| Oral tentacles - - - | - | - - - | - - |
| Aboral tentacles 1 3.3–4.3 3.9 ± 0.3 Hydranth 1 3.5–4.4 3.9 ± 0.3 | 18 7 | 2.3–3.2 2.8 ± 0.2 18 2.5–2.9 2.7 ± 0.2 7 | 1.35–1.43 Numerous 1.41 –1.,51 Few |
| Hydrocaulus 1 3.6–4.6 4.0 ± 0.3 | 7 | 2.5–3.2 2.9 ± 0.2 7 | 1.41–1.44 Numerous |
| Bermuda (ROMIZ B165) Basitrichous isorhizae | |||
| Oral tentacles 1 6.6–7.5 7.0 ± 0.31 | 10 | 2.0–2.4 2.2 ± 0.15 10 | 3.14–3.38 Few |
| Aboral tentacles 1 6.2–7.4 6.7 ± 0.32 Hydranth 1 6.1–8.1 7.1 ± 0.98 | 30 3 | 1.8–2.6 2.2 ± 0.23 30 2.3–2.7 2.5 ± 0.25 3 | 2.83–3.44 Few 2.68–2.95 Few |
| Hydrocaulus 1 5.7–7.2 6.4 ± 0.47 | 8 | 1.8–2.5 2.3 ± 0.21 8 | 2.90–3.16 Few |
| Desmonemes Oral tentacles - - - | - | - - - | - - |
| Aboral tentacles 1 3.1–4.2 3.3 ± 0.29 | 15 | 2.1–3.2 2.5 ± 0.24 15 | 1.34–1.45 Numerous |
| Hydranth 1 3.2–3.7 3.3 ± 0.18 Hydrocaulus 1 3.2–3.8 3.4 ± 0.28 | 7 6 | 2.5–2.8 2.7 ± 0.15 7 2.5–2.8 2.6 ± 0.11 6 | 1.28–1.31 Few 1.27–1.35 Numerous |
TABLE 7. Comparisons of dimensions of desmoneme and basitrichous isorhiza nematocysts of Zyzzyzus calderi and Zyzzyzus warreni. Data from the literature. Minimum and maximum values in bold and grey cells. All data in micrometers.
| Length | Width | |||
|---|---|---|---|---|
| Desmonemes | min. | max. | min. | max. |
| Z. calderi Petersen (1990) (Bermuda) | 3.7 | 4.2 | 2.8 | 2.9 |
| Z. warreni Petersen (1990) (Bermuda) | 4.5 | 6.3 | 3.6 | 3.6 |
| Z. warreni Migotto & Silveira (1987) (Brazil) | 2.6 | 4.6 | 2.6 | 4.6 |
| Z. warreni This study (Brazil) | 3.3 | 4.7 | 2.2 | 3.8 |
| Z. warreni Millard (1975) (South Africa) | 4.5 | 6.3 | 3.6 | 3.6 |
| Z. warreni This study (South Africa) | 3.3 | 4.6 | 2.3 | 3.2 |
| Z. warreni Calder (1988) (Bermuda) | 3.7 | 4.2 | 2.8 | 2.9 |
| Z. warreni This study (Bermuda) | 3.1 | 4.2 | 2.1 | 3.2 |
| Basitrichous isorhizae | ||||
| Z. calderi Petersen (1990) (Bermuda) | 6.4 | 7.3 | 1.9 | 2.1 |
| Z. warreni Petersen (1990) (Bermuda) | 8.1 | 9.0 | 3.6 | 3.6 |
| Z. warreni Migotto & Silveira (1987) (Brazil) | 3.3 | 7.9 | 1.3 | 4.0 |
| Z. warreni This study (Brazil) | 5.7 | 7.6 | 1.5 | 3.2 |
| Z. warreni Millard (1975) (South Africa) | 8.1 | 9.0 | 3.6 | 3.6 |
| Z. warreni This study (South Africa) | 5.7 | 7.6 | 1.8 | 3.5 |
| Z. warreni Calder (1988) (Bermuda) | 6.4 | 7.3 | 1.9 | 2.1 |
| Z. warreni This study (Bermuda) | 5.7 | 8.1 | 1.8 | 2.7 |
TABLE 5. Comparison of measurements of morphological characters of Zyzzyzus warreni, in paratypes from South Africa and specimens from Bermuda and Brazil.
| South Africa | Bermuda | Brazil | |||||
|---|---|---|---|---|---|---|---|
| Hydrorhiza | x ± SD Range (in Μm) (in Μm) | n | x ± SD (in Μm) | Range (in Μm) | n | x ± SD Range (in Μm) (in Μm) | n |
| Number of pro- cesses | - - | - | 3± 1 | 1–5 | 5 | - - | - |
| Length | - - | - | 2632±1129 | 1819–4397 | 5 | 2632 ± 1129 1819–4397 | 5 |
| Diameter | - - | - | 881±275 | 426–1413 | 23 | 881±275 426–1413 | 23 |
| Hydrocaulus |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Zyzzyzus warreni Calder, 1988
| Campos, Carlos J. A., Marques, Antonio C. & Migotto, Alvaro E. 2007 |
Zyzzyzus calderi
| Calder 1993: 66 |
| Petersen 1990: 180 |
Zyzzyzus warreni
| Migotto 1996: 25 |
| Calder 1988: 49 |
solitarius
| Bandel 1987: 39 |
Corymorpha solitaria
| Kramp 1933: 12 |
Zyzzyzus solitarius
| Migotto 1987: 104 |
| Wedler 1986: 72 |
| Petersen 1979: 121 |
| Millard 1975: 40 |
| Millard 1974: 2 |
| Kramp 1949: 198 |
| Stechow 1921: 249 |
Tubularia solitaria
| Kruijf 1977: 19 |
| Millard 1957: 179 |
| Warren 1908: 280 |
| Warren 1906: 83 |