Gyrodactylus jalalii, Vanhove & Boeger & Bukinga & Volckaert & Huyse & Pariselle, 2012
|
publication ID |
https://doi.org/ 10.5852/ejt.2012.30 |
|
publication LSID |
lsid:zoobank.org:pub:489A6BC2-795C-4945-A642-440D4F92A126 |
|
DOI |
https://doi.org/10.5281/zenodo.3859001 |
|
persistent identifier |
https://treatment.plazi.org/id/A89D45DC-7CA2-4807-AF2E-E34F9A9BB060 |
|
taxon LSID |
lsid:zoobank.org:act:A89D45DC-7CA2-4807-AF2E-E34F9A9BB060 |
|
treatment provided by |
Valdenar |
|
scientific name |
Gyrodactylus jalalii |
| status |
sp. nov. |
Gyrodactylus jalalii View in CoL sp. nov.
urn:lsid:zoobank.org:act:A89D45DC-7CA2-4807-AF2E-E34F9A9BB060
Etymology
The species epithet, jalalii , honours prof. dr. Behiar Jalali Jafari (1953-2010) (obituary in Shamsi 2010). He was a researcher in aquatic animal health and fish parasitology at the Veterinary Department of the Islamic Azad University ( Iran) and a keen student of monogeneans. The authors express the hope that this patronym might serve as an indication for the respect and appreciation this kind man enjoyed from his colleagues.
Type material examined
Thirty-one specimens, twenty of which (ethanol-preserved) were used for measurements. The holotype ( MNHN HEL301 ) and paratypes ( MNHN HEL302 – HEL305 ) are deposited in the Muséum National d’Histoire Naturelle (Paris, France). Paratypes are deposited in the Natural History Museum (London, United Kingdom) ( NHMUK 2012.9.10.1–2012.9.10.2), the Royal Museum for Central Africa (Tervuren, Belgium) ( MRAC MT: 37711–37713), the Harold W. Manter Laboratory of Parasitology (Lincoln, Nebraska) (HWML-49758) and the United States National Parasite Collection ( Beltsville , Maryland) ( USNPC 106050 ).
Type host
Iranocichla hormuzensis Coad, 1982 (Teleostei, Perciformes , Cichlidae ).
Type locality
Mehran River, Persian Gulf Basin (2009).
Infection site
Gill filaments, fins, eye.
Description
(measurements in micrometres (µm) and angles in degrees (°); average ± standard deviation, followed by range and number of measurements in parentheses).
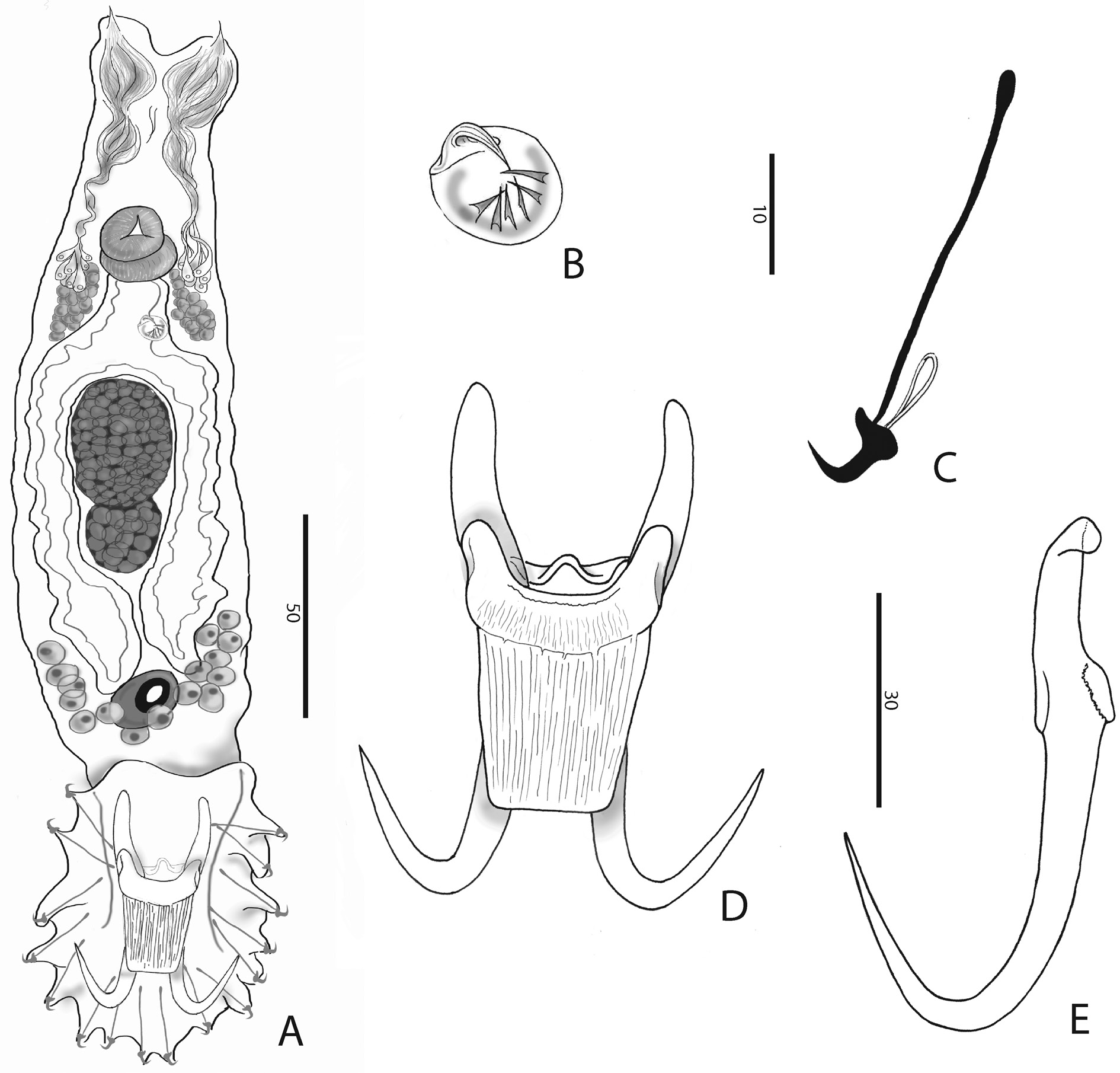
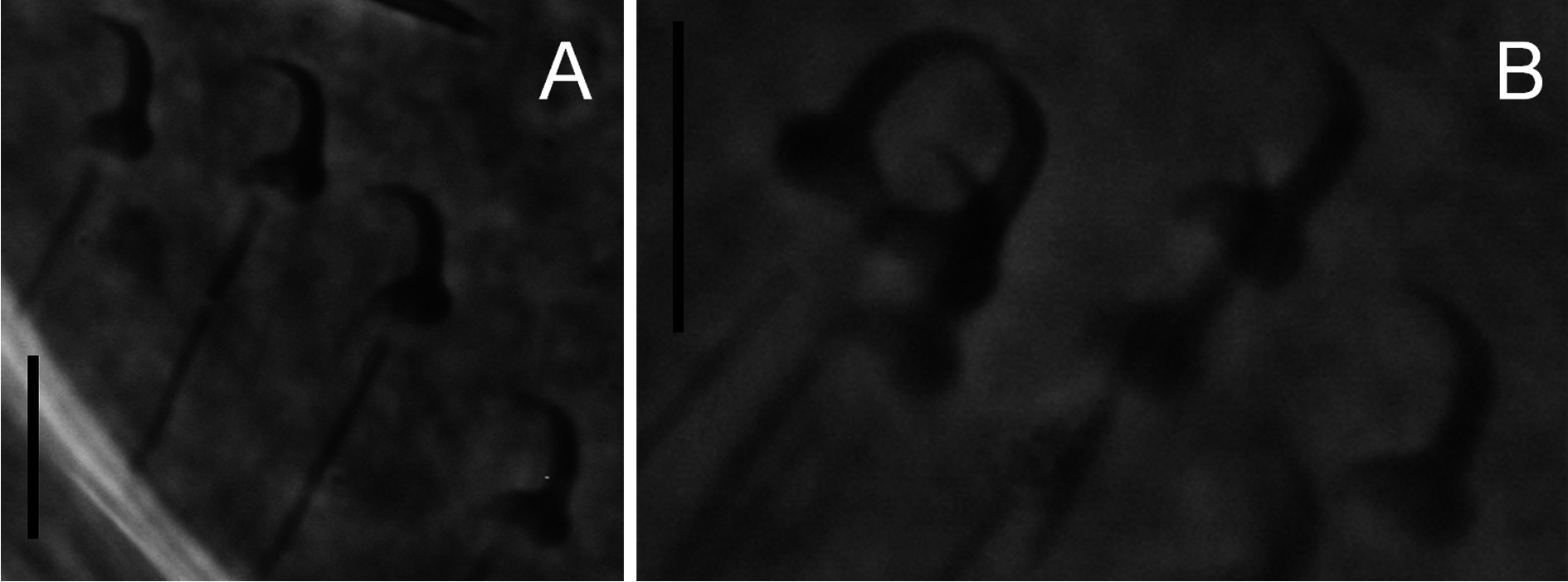
Body ( Fig. 1A View Fig ) fusiform, 361.7 ± 64.4 (294.7-528.9; n = 13) long; greatest width 128.1 ± 21.3 (98.8- 164.0; n = 13). Two head organs provided with single spicule each. Cephalic glands lateral to pharynx. Pharynx composed of two tandem, muscular bulbs. Oesophagus short. Digestive glands lateral to oesophagus. Caeca two, non-confluent, reaching level of germarium. Male copulatory organ (MCO) ( Fig. 1B View Fig ) armed with a broad-based, robust, recurved, apical spine 5.7 ± 1.1 (4.5-7.6; n = 8) long, 5-7 smaller flanking spines in a single row, becoming more slender from the terminal over the subterminal towards the median ones (terminology of García-Vásquez et al. 2007). Testis dorsal to germarium. Germarium immediately posterior to uterus. Uterus with up to 2 embryos. Unicellular glands lateral to terminations of caeca, posterior to germarium. Haptor elongate ( Fig. 1A, D View Fig ). Anchor (hamulus) ( Fig. 1E View Fig ) 79.9 ± 4.4 (70.4-86.3; n = 20) long; point 33.9 ± 2.3 (28.3-38.4; n = 19) long; base (superficial root) 28.2 ± 3.9 (19.7-33.1; n = 20) long; deep root knob-like; groove proximally at the base of the anchor, serving as articulation to superficial (ventral) bar; shaft 49.5 ± 2.2 (45.6-55.0; n = 19) long, proximally 10.2 ± 0.8 (8.7-11.9; n = 20) wide, distally 5.7 ± 0.8 (4.5-7.4; n = 19) wide; point sharply curved, with aperture 30.5 ± 2.5 (26.2-35.4; n = 20), aperture angle 42.7 ± 3.2 (36.9-47.3; n = 19), inner aperture angle 46.9 ± 5.3 (30.1-53.3; n = 19); inner curve length 1.9 ± 0.8 (0.7-3.3; n = 18) with point curve angle 9.5 ± 4.5 (4.2-22.9; n = 18). Ventral bar ( Fig. 1D View Fig ) 33.5 ± 2.5 (29.8-37.7; n = 20) wide, 44.1 ± 3.9 (36.4-49.8; n = 20) long; anterior bilateral processes slender, pronounced and 6.0 ± 1.1 (4.2-7.8; n = 20) long with process to mid-length 10.4 ± 1.7 (7.5-14.8; n = 20). Median portion of ventral bar 8.3 ± 1.2 (5.7-9.8; n = 20) long; shield (ventral bar membrane) 25.4 ± 3.3 (19.8-30.1; n = 20) long, subrectangular, clearly striated in much the same way as bar proper. Deep (dorsal) bar 23.0 ± 2.8 (18.9- 29.9; n = 20) wide, medially constricted and at connection to deep root of anchors. Marginal hook ( Figs 1C View Fig , 2A, B View Fig ) 31.8 ± 4.1 (25.8-43.5; n = 20) long, shank with small distal bulb, 26.9 ± 4.1 (22.1-40.0; n = 20) long; sickle (hooklet) 5.4 ± 0.3 (4.5-6.1; n = 20) long, 4.3 ± 0.4 (3.6-5.1; n = 20) wide proximally, 4.5 ± 0.5 (3.8-5.3; n = 20) distally; toe depressed, 2.0 ± 0.4 (1.4-3.0; n = 20) long; convex platform; concave base; round keel; point of sickle proper as long as shaft, forming an angle of about 90 ° from each other; aperture 5.2 ± 0.5 (4.5-6.4; n = 20); instep/arch height 0.6 ± 0.1 (0.4-0.8; n = 20).
Remarks
In comparison with congeners parasitizing cichlids, the striated ventral bar proper and shield, as well as the conspicuous ventral bar processes, seem most reminiscent to G. zimbae Vanhove, Snoeks, Huyse & Volckaert, 2011 . However, the anterolateral processes of the ventral bar of G. zimbae are more earshaped. In G. zimbae , the ventral bar shield is slender and rounded and the hooklet lacks an arched base (versus subrectangular shield and concave hooklet base in G. jalalii sp. nov.). Other cichlid Gyrodactylus with relatively large ventral bar processes include G. shariffi Cone, Arthur & Bondad-Reantaso, 1995 and G. yacatli García-Vásquez, Hansen, Christison, Bron & Shinn, 2011 . Just like in G. jalalii sp. nov., point and shaft of their hooklet sickle are at a right angle. These species are easily distinguished from G. jalalii sp. nov. by the smaller size of their haptoral sclerites and the ventral bar in particular (e.g., anchor 47.5 and 48.4 long, ventral bar shield 14.4 and 8.5 long, in G. shariffi and G. yacatli respectively) ( García-Vásquez et al. 2011). It should be noted, however, that these two species were described from cultured Oreochromis niloticus (Linnaeus, 1758) in the Philippines, resp. Mexico. The authors describing G. yacatli consider accidental infection or host switch a more likely scenario than an African origin ( García-Vásquez et al. 2011). Hence, G. zimbae seems to be the most comparable cichlid parasite whose natural distribution is certainly African.
Comparison to Palearctic congeners followed Pugachev et al. (2009). The rather large ventral bar processes, in combination with the length of the marginal hooks, and MCO armed with one large apical spine and one row of smaller spines of similar size, resemble the morphology of G. ophiocephali Gussev, 1955 from Channa argus (Cantor, 1842) ( Perciformes : Channidae ) and Cyprinus carpio Linnaeus, 1758 ( Cypriniformes , Cyprinidae ), and to G. tokobaevi Ergens & Karabekova, 1980 from Gymnodiptychus dybowskii (Kessler, 1874) ( Cypriniformes , Cyprinidae ). However, in G. ophiocephali and G. tokobaevi , the processes are longer than the ventral bar proper (median length, i.e., without the shield), which is not the case in G. jalalii sp. nov. Elongate antero-lateral processes, albeit not longer than the ventral bar proper, are also found in G. hrabei Ergens, 1957 and G. mariannae Winger, Hansen, Bachmann & Bakke, 2008 , parasites of Cottus Linnaeus, 1758 spp. ( Scorpaeniformes , Cottidae ). These species, however, have a comparatively shorter anchor root than G. jalalii sp. nov. The longitudinal striae on the ventral bar shield as well as an overlap in size of anchor and marginal hook are reminiscent of G. lotae Gussev, 1953 from Lota lota (Linnaeus, 1758) ( Gadiformes , Lotidae ). This species can be distinguished from G. jalalii sp. nov. because the new species has blunter and larger ventral bar processes, and a marginal hook sickle toe which joins smoothly into the platform, whereas this transition leaves a sharp “bump” in the platform in G. lotae .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |