Liphanthus centralis Mir Sharifi & Packer, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4645.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:01C0687D-D282-4E0C-8C3E-C2E70956C493 |
|
DOI |
https://doi.org/10.5281/zenodo.5942957 |
|
persistent identifier |
https://treatment.plazi.org/id/CA74085B-1BEA-42A4-AD12-BA43AE76AADA |
|
taxon LSID |
lsid:zoobank.org:act:CA74085B-1BEA-42A4-AD12-BA43AE76AADA |
|
treatment provided by |
Plazi (2019-07-22 08:39:23, last updated 2024-11-26 22:06:00) |
|
scientific name |
Liphanthus centralis Mir Sharifi & Packer |
| status |
sp. nov. |
Liphanthus centralis Mir Sharifi & Packer , sp. nov.
urn:lsid:zoobank.org:act:CA74085B-1BEA-42A4-AD12-BA43AE76AADA
Figs. 7–9 View FIGURES 4–9 , 41–51 View FIGURES 41–47 View FIGURES 48–51 , 139 View FIGURES 139–141 , 167 View FIGURES 165–168 , 171–172 View FIGURES 169–172 , 177–178 View FIGURES 177–180 , 183–184 View FIGURES 181–184 .
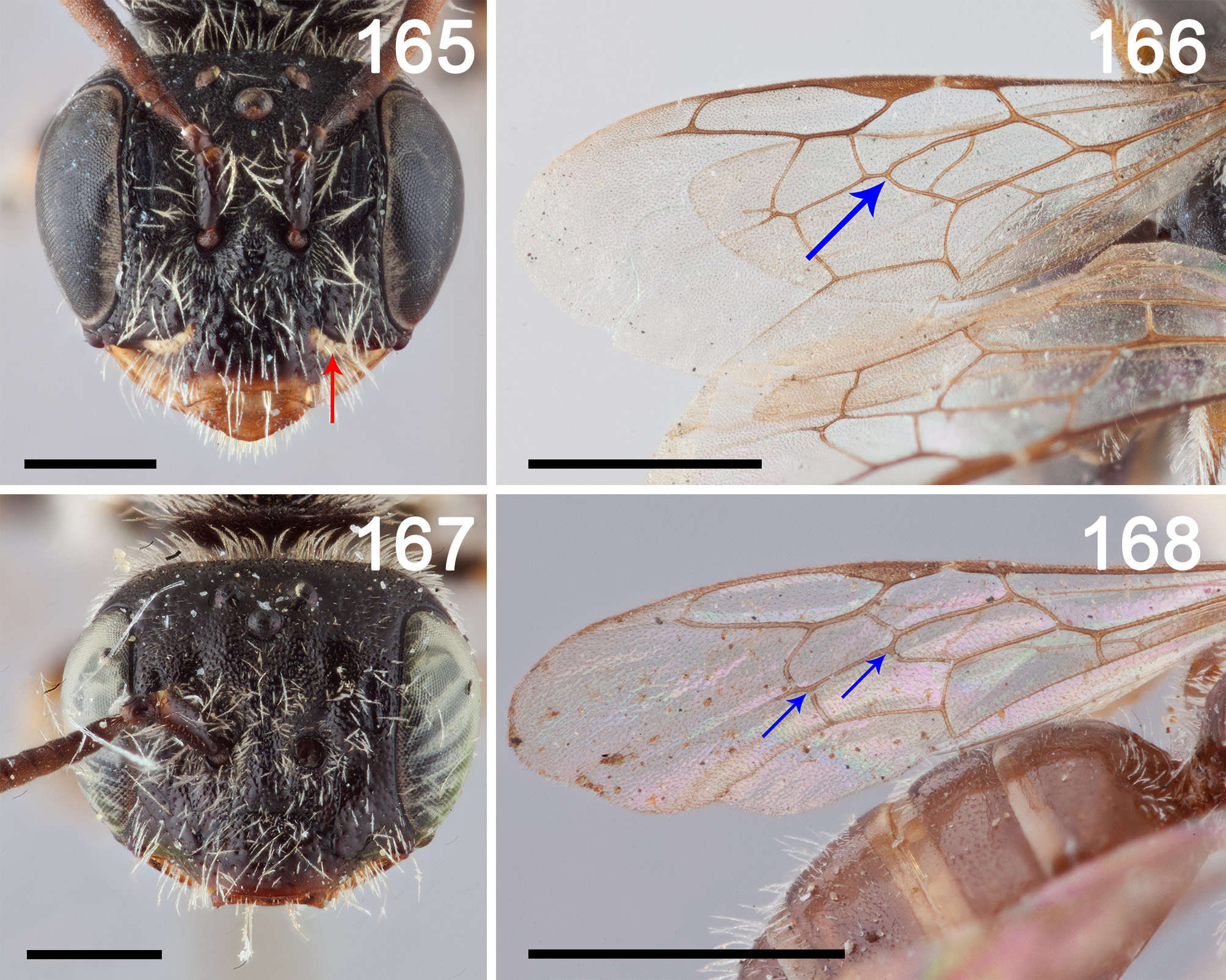
Diagnosis: Males of this species can be differentiated from all others of the genus except L. discolor and L. domeykoi by the combination of two submarginal cells, pronotal lobe yellow, metatibial spurs straight, S2 and S3 with posterior margin straight ( Fig. 44 View FIGURES 41–47 ). It can be differentiated from these two species by IAD equal to AOD (16:16) ( Fig. 42 View FIGURES 41–47 ) and face above antenna ( Fig. 43 View FIGURES 41–47 ) and mesoscutum weakly imbricate, shiny whereas IAD> AOD in both the other species (15:10 and 19: 16 in L. discolor and L. domeykoi respectively) both of which have the face and mesosoma minutely tuberculate (as in Fig. 1 View FIGURES 1–3 ) and dull ( Figs. 32 View FIGURES 31–33 & 35 View FIGURES 34–40 ). Females of this species can be differentiated from all others in the genus by the combination of two submarginal cells, pronotal lobe yellow, metatibial spurs straight ( Fig. 50 View FIGURES 48–51 ), clypeus dark ( Fig. 49 View FIGURES 48–51 ) and face and mesoscutum distinctly and densely punctate (i~d) ( Figs. 49 & 50 View FIGURES 48–51 ). The most similar species in the female is L. tregualamensis sp. nov. which shares the first four characteristics but is sparsely and obscurely punctate ( Figs. 109 & 111 View FIGURES 109–111 ).
Description. Holotype Male: Dimensions: Approximate body length: 4.69mm; head width: 1.44mm, wing length: 2.96mm, intertegular width: 0.78mm.
Coloration: Black to dark brown with following parts yellow: labrum, mandible (apex orange-red), clypeus, lower paraocular area from just above midlength of compound eye, subantennal sclerite (except extreme base), pronotal lobe, broad apical ring on all femora, all tibiae (except brown mark on anterior surface of protibia and posterior surface of mesotibia), all tarsi (except all tarsomeres 4 and 5 yellow-brown). Ventral surface of F3–F11, T6–T7 and S6 red-brown.
Sculpture: Microsculpture absent on whole body except as stated otherwise. Basal 2/3 of clypeus and subantennal sclerite punctures minute, dense, i≤d, apical 1/3 of clypeus punctures larger, irregularly spaced, i=1–3d; lower paraocular area punctures small irregularly spaced, i=1–4d above, larger, more evenly spaced below, i=1–2d; upper paraocular, frontal and vertexal areas punctures small, dense, i<d, increasing in size from above antennal sockets to vertexal area; genal area punctures small, moderately dense, i=1–1.5d, hypostomal area punctures small, scattered. Mesoscutum moderately densely punctate, i=1–1.5d; scutellum punctures variable in size, and spacing, i=0.5–2d; metanotum punctures small, i~d; metapostnotum sparsely rugulose medially, minutely punctate laterally, punctures moderately sparse, i=1.5–2d; hypoepimeral area moderately densely to moderately sparsely punctate, i=1–2d; mesopleuron punctures small, irregularly spaced, i=0.5–3d; metapleuron impunctate, coarsely imbricate below, lacking microsculpture above; sides of propodeum weakly imbricate. Metasomal terga punctures deep distinct dense, i<d except towards apex of T4 and T 5 i =1–2d, T 6 i =1–3d shallow on T7; apical impressed areas impunctate; metasomal sterna strongly imbricate, dull; sparsely and obscurely punctate, i>2d.
Pubescence: White, somewhat plumose and short; ≤1.5 MOD on head, sides of mesosoma and scutellum except < MOD on genal and vertexal areas and <0.7 MOD and pale brown on frontal area; mesoscutum hairs plumose, dense and short ≤0.5 MOD anteriorly and posteriorly, orange-brown tinged along admedian line, posterior to notaulus and on anterior portion of posterior patch, posterior patch hairs subappressed, laterally oriented, few erect longer hairs, ~1.5 MOD along anterior margin; metanotum erect hairs up to 2.3 MOD; metapostnotum dorsal surface hairs somewhat plumose ~ MOD. Metasomal terga disc hairs simple, increasing in length from <0.5 MOD on T1 to 1.1 MOD on T7; weak subapical hair bands of short plumose hairs ~0.7 MOD on T1–T3, simple longer on T4–T6 <1 MOD; T7 largely covered in branched, posteromedially oriented, long <1.3 MOD hairs. Sterna with minute hairs, dense on S2, longer posteriorly on S3 ~0.3 MOD, S4 with subapical row of bristles, ~0.5 MOD, briefly interrupted medially.
Structure: Head: ~ 1.15 X as wide as long (93:79). Mandible 1.9 X as long as basal depth (45:24), with tuft of branched hairs ventrally at midlength, branches long restricted to one side of rachis, outer ridge lamellate. Labrum rectangular twice as broad as long (30:15). Clypeus ~1.6 X as wide as long (59:36); apicolateral margin slightly concave in frontal view, weakly convex apicomedially. Outer subantennal suture weakly curved, inner suture straight, subantennal sclerite widest near midlength; epistomal suture weakly curved between inner subantennal sutures. Anterior tentorial pit just below junction of outer subantennal and epistomal sutures. Frontal line narrowly and mostly weakly depressed, punctate. IAD subequal to AOD (17:16). Inner margins of compound eyes convergent below, UOD:LOD 60:45. Facial fovea somewhat comma-shaped, lower margin above junction between dark and yellow parts of upper paraocular area, somewhat convergent to inner margin of compound eye below, length to width: 10:4. IOC: OOC 20:26. Scape ~ 1.8 X as long as greatest width (23:13), subequal to pedicel and F1 combined (22); pedicel length subequal to width (9:10), F1 longer than wide (13:10). F2 shorter than wide (9:11), remaining flagellomeres slightly longer than wide except F11~1.7 X as long as wide (20:12).
Mesosoma: Mesoscutum ~1.1 X as wide as long (76:70); length of scutellum: metanotum: metapostnotum: 28:22:11. Marginal cell slightly shorter than distance between its apex to wing tip (66:70). Metatibial spurs straight, posterior spur longer than anterior. Posterior metatarsal claw with axe-shaped tooth, anterior claw with tooth broad; claws of other tarsi cleft.
Metasoma: Broadest at midlength of T3; terga not strongly ventromedially reflexed towards sides; pygidial plate absent, S2–S4 posterior margins narrowly, weakly concave medially, S4 with row of posterolaterally oriented robust hairs, narrowly interrupted medially; S6 broadly shallowly concave apicomedially, premarginal line extended into numerous posteriorly oriented narrow processes, with few posteriorly oriented hairs towards sides. S7 apodeme long and narrow, anterior margin mostly transverse, apex sinuate strongly curved anteriorly; apical lobe short, wider than long, subtriangular with sparse short posteriorly oriented hairs, hairs shorter than length of lobe. S8. Anterior margin strongly convex and broad, short spiculum anteriorly; lateral margins briefly concave anterior to short recurved lateral lobe; lateral lobe small, apex acute; apical lobe narrowing towards broader apex with sides rounded and apical margin weakly concave, short hair posteriorly. Gonocoxa anterodorsal margin strongly convex forming ~ right angle medially, lateral margins diverging posteriorly, medial margin almost straight except sinuate apically; gonostylus distinctly separated from gonocoxa by weakly sclerotized band, anteroventrally recurved, lateral margin convex, medial margin strongly concave with subapical tooth, densely hairy, hairs longest towards apex of lateral margin; penis valve small, narrow. Endophallus complex in structure with strongly sclerotized ventral portion apex broadly rounded, disc-like, the disc bears two pairs of membranous flaps, one small and ringing the anterior portion of the disc-like apex, the other pair large, surrounding the disc and smaller flap anteriorly and extending laterally and apically well beyond the apex of the penis valve.
Allotype Female: As in male except for usual sexually dimorphic features and as follows:
Dimensions: Approximate body length: 5.38mm; head width: 1.62mm, wing length: 3.54mm, intertegular width: 0.95mm.
Coloration: Black to dark brown with following parts yellow: apical ring on all femora, pro- and mesotibiae anterobasally. Following parts yellow-brown: labrum, mandible (except apex red and base suffused with brown), anterior spot on tegula, rest of tibiae, all tarsi, apical impressed areas of metasomal terga. Metasomal terga and metasomal sterna orange-brown.
Sculpture: Microsculpture entirely absent except very weak on apical impressed areas of metasomal T1-T3 and distinct on apical impressed area of T4, weak on metasomal sterna. Clypeus punctures variable in size and spacing, i=0.1–5d, sparsest towards apex; lower paraocular area punctures variable in size, moderately dense, i=0.2–1d; frontal area punctures small, shallow and sparse, i≥d, in supraantennal portion; hypostomal area punctures larger and denser, i=1–3d; mesoscutum and scutellum punctures irregular in size and spacing, i=0.5–3d and i=0.5–4d respectively; metapostnotum dorsal surface impunctate medially, elsewhere punctures small, i=1–2d; hypoepimeral area moderately densely punctate, i=0.5–1.5d. T1 moderately densely punctate, i=0.5–1.5d; T2–T3 moderately densely punctate anteriorly, moderately sparsely punctate posteriorly, T4 punctures smaller, i=0.5–2d; T5 punctures anteriorly small and dense i~d, posteriorly large and sparser, i=1–3d; metasomal sterna sparsely punctate anteriorly, i>2d, moderately sparsely punctate posteriorly, i=1.5–3d, denser on S4 and S 5 i =1–2d.
Pubescence: Longest hairs often longer than in male, mostly ≤2 MOD, ≤3 MOD on sides of mesosoma ; scopal hairs strongly curved, simple, ~2.5 MOD. Prepygidial fimbria pale brown, dense and ~2–2.5 MOD.
Structure: Head: 1.2 X as wide as long (77:64). Mandible 2.1 X as long as basal depth (52:25), apex rounded. Labrum less than twice as broad as long (34:20), raised area sides convergent below, apical margin weakly biconvex. Clypeus ~ 2.5 X as wide as long (90:37). Anterior tentorial pit just above junction of outer subantennal and epistomal sutures. Frontal line a row of slightly depressed punctures, briefly deep below midlength. IAD<AOD (22:24). Inner margins of compound eyes an almost continuous curve, UOD not measurable, least interorbital distance at level of antennal socket. Facial fovea parallel-sided, somewhat convergent towards inner margin of compound eye below, length to width: 15:4. IOC: OOC 23:30. Scape>2.6 X as long as greatest width (35:13), longer than pedicel and F1 combined (24). Pedicel and F1 as long as wide, both 12:12; F2 shorter than wide (8:12), remaining flagellomeres with length and width subequal except F10 ~1.5 X as long as wide (21:14).
Mesosoma: Mesoscutum shorter than wide, 81:91. Length of scutellum: metanotum: metapostnotum:32:22:14. Marginal cell slightly shorter than distance between its apex to wing tip (80:85). Tarsal claws with short teeth.
Metasoma: Metasoma widest at midlength of T3, sterna unmodified. Pygidial plate triangular, sides straight, forming angle of ~70˚, apex moderately rounded, broadly raised medially.
Material studied: Holotype male, allotype female, 25 male and 23 female paratypes as follows: Holotype male, allotype female and 2 male paratypes: CHILE, Region Metro {politana}, Valle Nevado, 2596m, -33.34111, -70.29497, 9.i.2009, L. Packer. One paratype male and one paratype female: CHILE, Region Metro {politana}, Farellones, -33.35627, -70.32478, 2179m, 9.i.2009, L. Packer. Six males: CHILE, Valparaiso: W of Cuesta Colliguay, x.5.1969, Rozen & Peña. Five males, one female: CHILE, Region V, Caleu, NW of Tiltil, 9.xi.1997, L. Packer. Eleven males and 21 females: CHILE, R.M, Chacabuco Caleu, nr. Cerro del Roble {= Cerro El Roble}, -33.0136, -70.983, 30.xi.2004, J. S. Ascher, A. Y. Kawahara, C. Espina. The holotype, allotype and paratypes from Valle Nevado and Farellones and paratypes from Caleu are at PCYU except one male at PUCV (the holotype and allotype will be sent to MNHN), the remaining specimens are at the AMNH GoogleMaps .
Etymology. The specific epithet refers to the geographic range of the species in Central Chile.
DNA barcodes. One full length (from the allotype) and one partial sequence are available for this species, they differ by 0.53% and the nearest neighbor is L. pilifrons Ruz & Toro 1983 from which the sequences differ by 10.08%, although L. centralis does not cluster with this species. Its BIN is AAV8032 and the one sequence on genbank noted by Packer and Ruz (2017) is KX820707 View Materials .
Comments. Treating it as if it had three submarginal cells, using the key in Ruz and Toro (1983) males of this species fail at couplet 10 which contrasts different forms of process on S2: L. centralis has no process on S2. Females fail at couplet 35 where they would key out to L. (Leptophanthus) anacanthus Ruz & Toro 1983 based on clypeal coloration but have the facial fovea shorter than the scape as for L. (Lpt.) alicahue Ruz & Toro 1983.
The specimens from Cerro El Roble and Caleu are from moderate altitude in the coastal mountain range and those from Cuesta Colliguay are from lower elevations further west, whereas those from Valle Nevado and Farellones are from the Andes range proper. We found no consistent differences among specimens between the two mountain ranges.
The structures associated with the penis of this species are remarkably complex. It seems as if the penis valves proper are quite simple, short and not curved ventrally towards the apex but the endophallus is considerably elaborated. No other Liphanthus known to us has such an elaborate endophallus.
This species has been collected in reasonably large numbers from areas that have long received considerable attention from melittologists yet with only one specimen found before 1997. The locality referred to on data labels as “Farellones” is one of steep and strongly curved roads (leading Monckton, 2016 to name a new species from this area as Chilicola curvapeligrosa ) where numerous interesting bees have been found including the aforementioned Chilicola Spinola 1851 species, Xeromelissa farellones (Toro and Moldenke) ( Toro and Moldenke, 1979) and close to where Eucerinoda gayi Michener and Moure 1957 was recently rediscovered ( Vivallo, 2009).
Monckton, S. K. (2016) A revision of Chilicola (Heteroediscelis), a subgenus of xeromelissine bees (Hymenoptera, Colletidae) endemic to Chile: taxonomy, phylogeny, and biogeography, with descriptions of eight new species. ZooKeys, 591, 1 - 144.
Packer, L. & Ruz, L. (2017) DNA barcoding the bees (Hymenoptera: Apoidea) of Chile: species disoversy in a reasonably well known bee fauna with the description of a new species of Lonchopria (Colletidae). Genome, 60, 414 - 430.
Ruz, L. & Toro, H. (1983) Revision of the bee genus Liphanthus (Hymenoptera: Andrenidae). University of Kansas Science Bulletin, 52, 235 - 299.
Toro, H. & Moldenke, A. (1979) Revision de los Xeromelissinae chilenos. Anales del Museo de Historia Natural de Valparaiso, 12, 95 - 182.
Vivallo, F. (2009) Notes on the bee genus Alloscirtetica Holmberg, 1909 in northern Chile with the description of two new altiplanic species and a key for the Chilean species of Eucerini (Hymenoptera: Apidae). Zootaxa, 2010, 16 - 30.
FIGURES 4–9. Apicomedial portion of genital capsule ventral view (unless otherwise stated) to show elaboration of the endophallus, 4: L. jenamro sp. nov. holotype, apex of extension indicated by red arrow, 5: L. molavi sp. nov. paratype to show broadly triangular sclerotized (lateral apex indicated by red arrow) portion of endophallus, 6: L.amblayensis sp. nov. holotype to show absence of elaboration; 7–9: L. centralis sp. nov. paratypes, 7: to show very complex structure of cleared structures as seen with light microscopy in ventral view, 8: detail of genitalia to show structure of apical portion of penis valve in uncleared material, oblique view. 9: ESEM to show details of apical portion of penis/penis valve oblique view, showing minutely tuberculate/papillate microstructure, red arrow points to the apex of the penis valve. Scale bar Figs. 4–8 0.125mm, Fig. 9 100 μm.
FIGURES 41–47. Liphanthus centralis sp. nov., male paratype: 41: lateral habitus, 42: head frontal view, 43: upper portion of face to show facial fovea (red arrow), 44: metasoma ventral surface to show straight posterior margins of S2 and S3, 45: S7, 46: S8, 47: genital capsule. Scale bars Figs. 41–42 & 44 1mm, Fig. 43 0.25mm, Figs. 45–47 0.125mm.
FIGURES 48–51. Liphanthus centralis sp. nov., female allotype: 48: lateral habitus, 49: head frontal view, 50: apex of tibia to show rather straight metatibial spurs, 51: mesosomal dorsum to show dense punctation. Scale bars Figs. 48–49 1mm, Fig. 50 0.25mm, Fig. 51 0.5mm.
FIGURES 139–141. Liphanthus sp. metasomal venters to show margin of S2:139: L. centralis, lacking apicomedian process (arrow), 140: L. bicellularis with acute, narrow apicomedian process (between arrows), 141: L. molavi with short, blunt and setose apicomedial process (between arrows). Scale bars 0.5mm.
FIGURES 165–168. 165: L. aliavenus face frontal view to show yellow marking (red arrow); 166: L. aliavenus forewing to show first recurrent vein entering first submarginal cell (blue arrow), 167: L. centralis face frontal view to show absence of basolateral marking on clypeus, Fig. 168: L. tregualemensis holotype forewing to show both recurrent veins entering second submarginal cell (blue arrows). Scale bars Figs. 165 & 167 0.5mm, Figs. 166 & 168 1mm.
FIGURES 169–172. 169: L. sapos metatibial spurs to show strong curvature; 170: L. jenamro to show IOD (red line)> OOD (blue line), 171: L. centralis metatibial spurs to show weak curvature, 172: L. centralis to show IOD (red line) <OOD (blue line). Scale bars Fig. 169 100μm, Figs. 170 & 172 0.5mm, Fig. 171 50μm.
FIGURES 177–180. 177: L. centralis head, frontal view to show absence of yellow marking on clypeus, 178: L. centralis dorsal view of posterior portion of mesosoma to show lack of surface sculpture on metapostnotum, 179: L. molavi head, frontal view to show yellow marking on clypeus, 180: L. molavi dorsal view of posterior portion of mesosoma to show surface sculpture on metapostnotum. Scale bars 0.5mm.
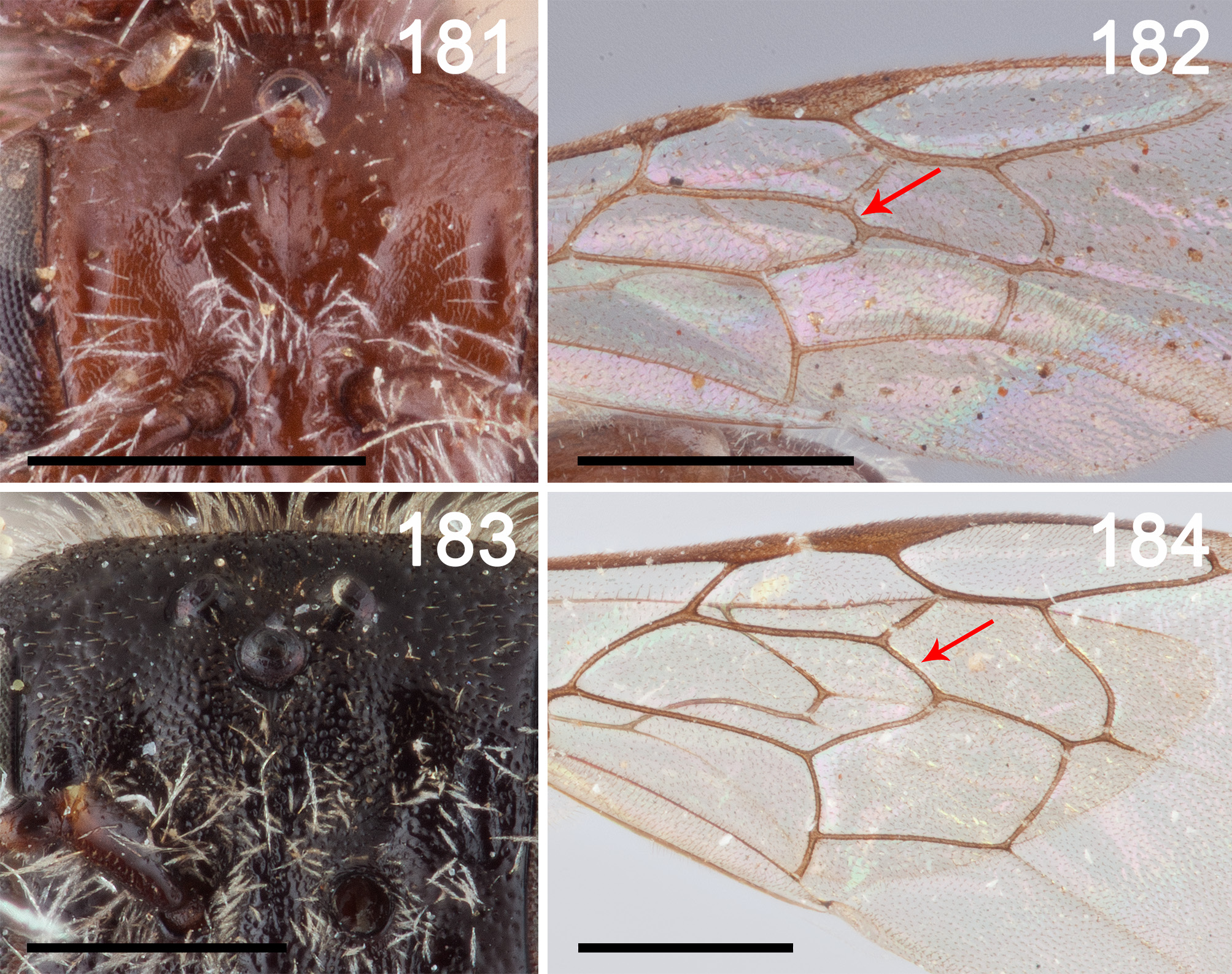
FIGURES 181–184. 181: L. tregualemensis frontal area of head frontal view to show sparse sculpture, 182: L. tregualemensis forewing to show relatively short separation of first recurrent vein and first submarginal crossvein (red line), 183: L. centralis frontal area of head frontal view to show dense punctation, 184: L. centralis forewing to show relatively long separation of first recurrent vein and first submarginal crossvein (red line). Scale bars 0.5mm.
FIGURES 1–3. Unusual morphological features of some Liphanthus species. 1: L. jenamro sp. nov. ESEM of frontal area of holotype male to show minutely and densely tuberculate microsculpture, 2: L. jenamro ESEM of frontal area of allotype female to show acinose (bracket) to minutely and densely tuberculate microsculpture, 3: L. jenamro mandibles of a paratype male to show tuft of specialized hairs at the junction of the outer ridge and ventral margin, note the long branches on one side of the rachis only. Scale bars Figs. 1–2 100μm, Fig. 3 0.25mm.
FIGURES 31–33. Liphanthus domeykoi sp. nov., holotype. 31: lateral habitus, 32: head frontal view, 33: posterior part of meso- and most of metasoma oblique ventral view to show yellow ventral surface of trochanters (red arrows) and concave and yellow metasomal sterna. Scale bars 1mm.
FIGURES 34–40. Liphanthus discolor sp. nov., holotype, 34: lateral habitus, 35: face frontal view, 36: upper paraocular area to show absence of facial fovea (arrow to area where this occurs in related species, compare to Figs. 22 & 43), 37: metasomal venter to show brownish colouration and reduced yellow on reflexed portions of terga, 38: S7, 39: S8, 40: genital capsule. Scale bars Figs. 34–35 & 37 1mm, Fig. 36 0.25mm, Figs. 38–40 0.125mm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
1 (by plazi, 2019-07-22 08:39:23)
2 (by ImsDioSync, 2019-07-22 08:47:47)
3 (by ImsDioSync, 2019-07-26 11:35:47)
4 (by ExternalLinkService, 2019-09-25 22:16:20)
5 (by ExternalLinkService, 2022-01-29 13:19:09)
6 (by ExternalLinkService, 2022-01-30 01:23:52)
7 (by ExternalLinkService, 2022-02-01 23:11:11)
8 (by plazi, 2023-10-30 17:01:22)