Rumex palustris Smith (1800: 394)
|
publication ID |
https://doi.org/10.11646/phytotaxa.587.2.5 |
|
DOI |
https://doi.org/10.5281/zenodo.7734689 |
|
persistent identifier |
https://treatment.plazi.org/id/395787C3-FF82-571C-01B5-C146C2DCF8EF |
|
treatment provided by |
Plazi |
|
scientific name |
Rumex palustris Smith (1800: 394) |
| status |
|
Rumex palustris Smith (1800: 394) View in CoL View at ENA
≡ Lapathum palustre (Sm.) Gray (1821: 275) View in CoL
≡ Rumex maritimus subsp. palustris (Sm.) Hooker (1870: 312) View in CoL .
= Rumex limosus Thuillier (1799: 182) View in CoL = Lapathum limosum (Thuill.) Renault (1804: 60) View in CoL ≡ Rumex maritimus subsp. limosus (Thuill.) Čelakovský (1871: 158) View in CoL ≡ Rumex maritimus var. limosus (Thuill.) Formánek (1886: 25) View in CoL .
= Rumex palustris var. nanus Bo View in CoL ̈nninghausen (1824: 108).
= Rumex uliginosus Gussone (1826: 151) View in CoL ≡ Rumex palustris subsp. uliginosus (Guss.) Arcangeli (1882: 585) View in CoL .
= Steinmannia flavovirens Opiz (1852: 93) View in CoL .
= Rumex laxiflorus Saint-Lager (1889: 709) View in CoL .
= Rumex maritimus ( Linnaeus (1753: 335) View in CoL View Cited Treatment ≡ Rumex palustris subsp. maritimus (L.) Bonnier & Layens (1894: 272). View in CoL
Description ( Figs. 1 View FIGURE 1 and 2A–I View FIGURE 2 ):— Herb annual, biennial or perennial, glabrous or rarely sparsely minute papillose, initially green, after flowering brown-reddish. Stem erect, sulcate, up to 150 cm high, branched below middle, occasionally near base. Leaves linear-lanceolate, ca. 7–30(–35) × (1.0–) 1.5–6.5 cm, apex acute, truncate to cuneate at base, margin entire and slightly undulate; basal leaves about 5–6 times longer than wide, blade lanceolate or lanceolateoblong, base truncate to broadly cuneate, with slender petiole ca. 2–14 cm long; cauline leaves lanceolate-linear gradually narrower and smaller, with shortly petiolate or subsessile ca. 0.5–1.0 cm long; ocrea fugacious, membranous. Inflorescence paniculate, arcuate branches, dense, at least 15 flowers in each whorl, reddish brown at maturity (initially green). Flowers bisexual; pedicels jointed at base, slightly longer or as long as the valves, ca. 2–6(–7) mm long, unequal, filiform, erect to curved, articulated near base or below middle (ca. 1/3–1/4 from base); outer perianth segments lanceolate, small, ca. 2.0–2.5 × 0.5–0.7 mm; inner tepals (or valves) rhombic-triangular or linguliform, longer than outers, ca. 3–4(–5) × (1.0–)1.5–2.0 mm, straight, apex acute, base truncate or broadly cuneate, reticulated veins on the surface, all valves distinctly denticulate at margin (tepales spiny margined) and tuberculate on the back; teeth subulatefiliform, 2–3 on each margins, bristle-like, straight, rarely curved, ca. 1.3–2.8 mm long, equaling or slightly longer than valve width; tubercles oblong, large, equal or subequal, ca. 2 × 0.5–0.7 mm, obtuse. smooth (striate in herbarium specimens), initially white-greenish, after fruiting brown-reddish; stamens 6, filaments filiform, thin, ca. 1.0–1.3(–1.5) mm long, anther oblong, ca. 1.0– 1.5 mm long, yellow; ovary ovate, with 3 (rarely 5) lateral furrows, apex acute, ca. 1–2 mm long, styles 3, filiform, short, ca. 0.5–0.7 mm long, pendant, stigmas 3, tufted. Achene ovate, sharply trigonous (rarely pentagonal), ca. 1.9–2.0(–2.1) × 0.9–1.0(–1.1) mm, bright brown to golden yellow.
Phenology:— Flowering and fruiting in May–June.
Chromosome number:— 2n = 40 and 60 ( Rechinger 1964, Freeman & Reveal, 2005).
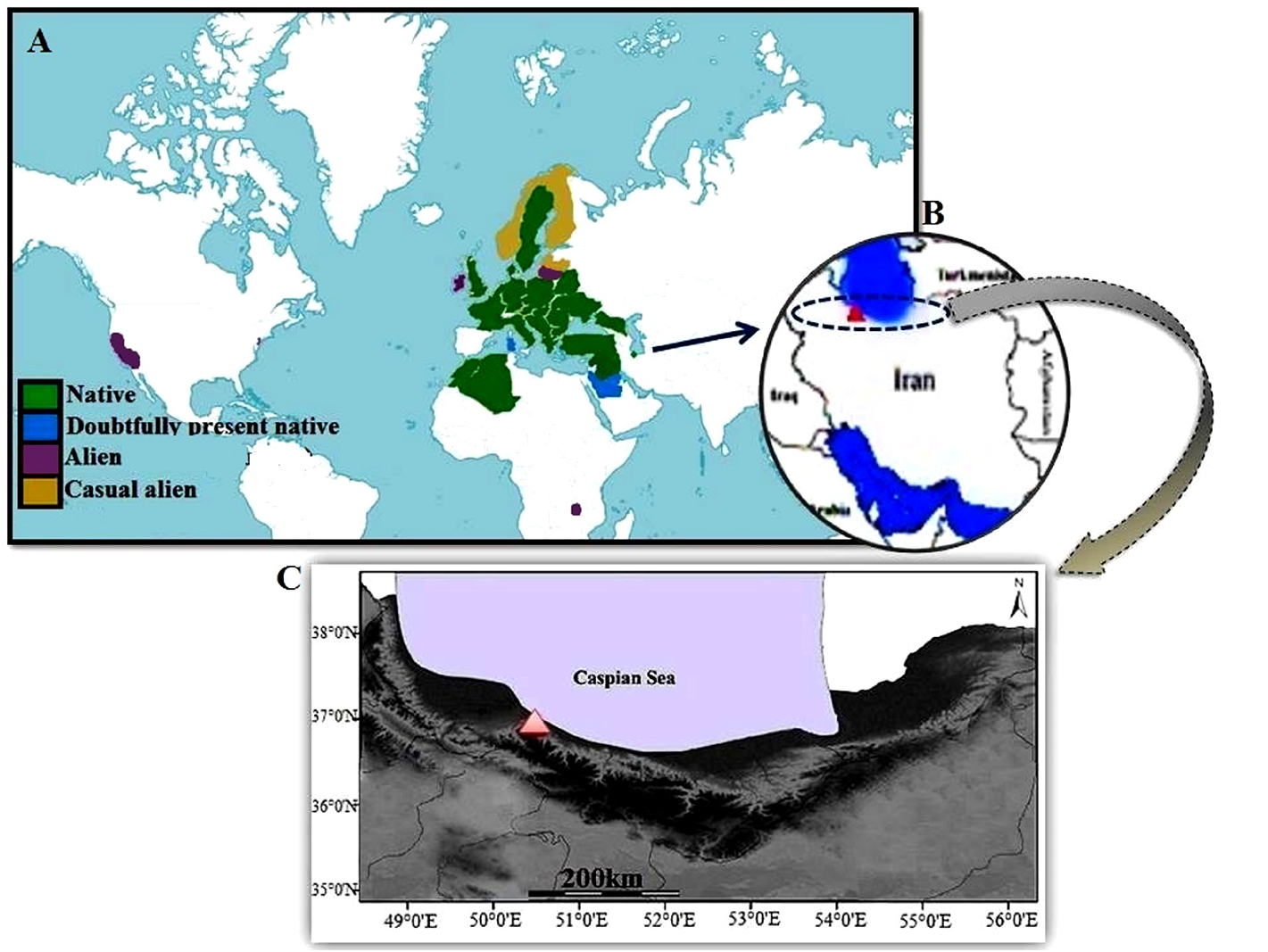
Distribution and habitat:— Rumex palustris grows on wet sand dunes and local dune slacks covered by seasonal fresh or brackish water in the south Caspian Sea coast, N. Iran ( Fig. 3 View FIGURE 3 ). This species is accompanied by other wetland and aquatic species, such as Nasturtium officinale Aiton (1812: 110) , Agrostis stolonifera Linnaeus (1753: 62) and Polypogon fugax Nees ex Steudel (1855: 184) .
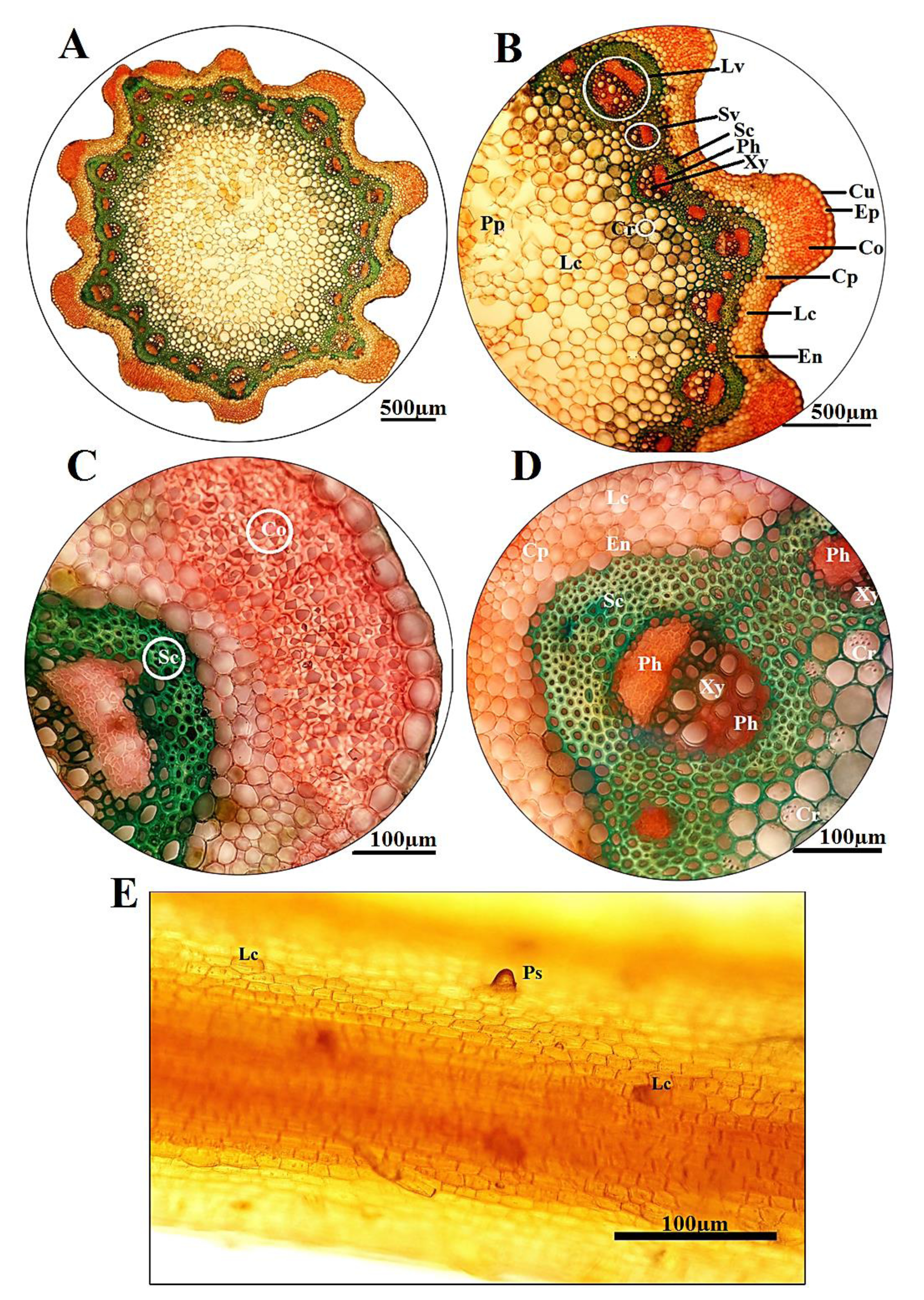
Anatomical observations:— Micrographs of stem anatomy in Rumex palustris are shown in Figs. 4A–E View FIGURE 4 . The main elements were determined to be taxonomically informative: cuticle, epidermis, collenchymatous zone, parenchymatous cortex (including laticifer cells), sclerenchymatous ring, vascular bundles and pith region (including laticifers and calcium oxalate crystals), which located from epidermis toward the central region in all transverse sections, respectively ( Figs. 4A and B View FIGURE 4 ). The stem outline of examined species was circular, along with several protuberances in the different sizes. The outer surface of cross-sections was also surrounded by a relatively thick cuticle and uni-seriate epidermis layer with rectangular cells ( Fig. 4B View FIGURE 4 ). Lamellar collenchyma consisting 5–10 layers was considerably observed at the ribs of stem, beneath epidermis ( Fig. 4C View FIGURE 4 ). The parenchymatous cortex was consisted of 5–7 cell layers in circularelliptic shapes, also laticifers located in this zone, but no crystal was seen. Underlying the distinct endodermis, vascular bundles were notably surrounded by 4–7 sclerenchymatous rings. All vascular bundles were arranged in one ring (ca. 35–40 in number), regular, in form of collateral for smaller bundles (ca. 16–20), as well as bicollateral for larger ones (ca. 20–23). The central region was completely filled with parenchymatous pith enclosing intercellular spaces, and also laticifers and some sand crystals ( Fig. 4D View FIGURE 4 ). In addition, the longitudinal sections of stem epidermal layer displayed sparsely short papilloses, along with laticifer cells on epidermis surface ( Fig. 4E View FIGURE 4 ).
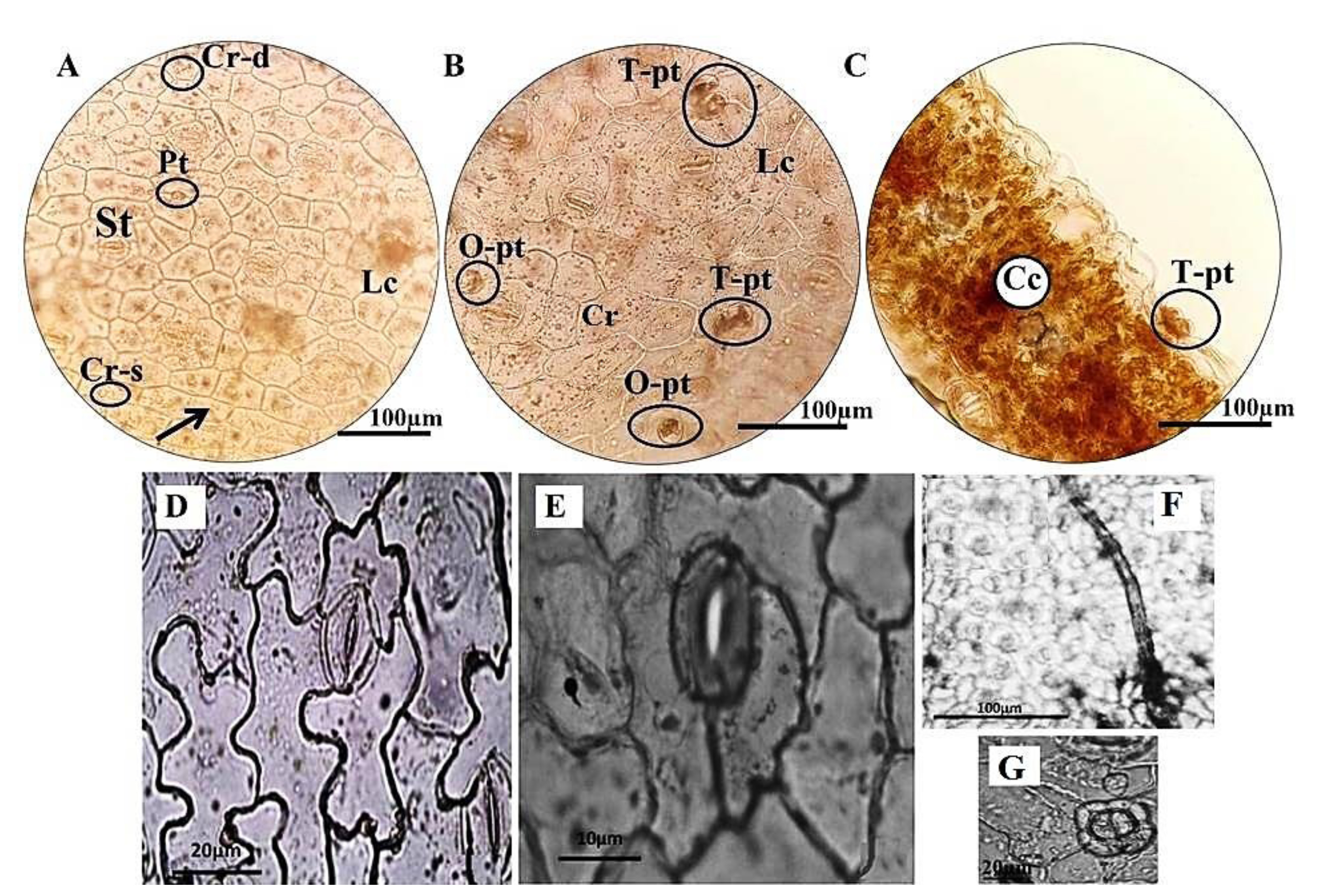
In the longitudinal sections of leaf, the epidermal layer consisted of polygonal cells (such as rectangular, pentagonal and hexagonal) with straight cell walls at both upper and lower surfaces. Anisosytic stomata, calcium oxalate crystals (such as sands and druses), laticifers and peltate trichomes (short stalked, peltate glands with one or two cells) were also revealed in the leaf epidermises ( Figs. 5A and B View FIGURE 5 ). Additionally, the leaf cross-sections proved the presence of peltate glands and crystalliferous cells in the epidermis and mesophyll, respectively ( Fig. 5C View FIGURE 5 ).
Also, anatomical findings of epidermis in Rumex palustris were compared with two close relatives, R. dentatus and R. maritimus ( Figs. 5D–G View FIGURE 5 ). The irregular epidermal cells with sinuate walls and both ansiocytic and pericytic stomata were detected in R. dentatus ( Fig. 5D View FIGURE 5 ), whereas R. maritimus exhibited the polygonal epidermis cells with smooth walls or nearly undulation and only pericytic stomata ( Fig. 5E View FIGURE 5 ). Moreover, indumentum type of epidermis surface considerably varied among species, ranging from peltate trichomes (with two cells) in R. maritimus to sessile glands and strigose and stellate trichomes (with four cells) in R. dentatus ( Figs. 5F–G View FIGURE 5 ).
Previous anatomical studies revealed that presence of epidermal trichomes, number of layers of cortex and collenchyma, type of collenchyma, number of sclerenchymatous rings, presence and position of laticifer cells, pith presence, crystals type, type of vascular bundles and number of vascular rings in stem, as well as, type of trichomes and stomata, cell shape, undulation details of cell wall and crystals type in leaf epidermis are practical and value characters in determination of Rumex taxa ( Yasmin 2009, Yasmin et al. 2010, Soleimani et al. 2014, Keshavarzi et al. 2018, Ulcay 2020, Paul & Chowdhury 2021).
In the current study, the above-mentioned characteristics were evaluated between R. palustris and other species of Rumex , focusing on its closest species, R. dentatus and R. maritimu s. Most of observed anatomical characteristics of stem such as the presence of layers of cortex, collenchyma and sclerenchyma, crystals and laticifers as well as pith region in R. palustris are in consistent with anatomical reports of other species of Rumex ( Soleimani et al. 2014, Keshavarzi et al. 2018, Mohamed & Azar 2020, Ulcay 2020). However, it can be distinguished from other members of Rumex using number of layers of cortex (5–7), collenchyma (5–10) and sclerenchyma (4–7), collenchyma type (lamellar), type and position of crystals (druses in pith and cortex) and type of vascular bundles (both collateral and bicollateral), showing the taxonomic importance of these characters. According to reported studies ( Soleimani et al. 2014, Keshavarzi et al. 2018), R. palustris noteworthy differs from R. dentatus by having 7 cortex layers, 4–6 collenchyma layers, 2–3 sclerenchymatous rings, annular collenchyma and only sand crystals and collateral vascular bundles.
In a comparative anatomy of epidermis between Rumex palustris (based on our data) and two its close species (using reported results), it differs from R. maritimus by type of stomata, crystal and peltate trichome ( Paul & Chowdhury 2021), and is also separated from R. dentatus based on cell shape and type of wall, crystal and trichome ( Yasmin 2009, Yasmin et al. 2010, Keshavarzi et al. 2018, Mohamed & Azar 2020, Paul & Chowdhury 2021). These anatomical differences of epidermis between R. palustris and its affinities were listed in Table 1 View TABLE 1 .
Taxonomic notes:— Based on our taxonomical results, Rumex palustris is considerably characterized by its height, size of leaves and petioles, shape of leaf base, articulation type of pedicels, shape and size of valves, number of teeth and tubercles, as well as size and color of achenes (see Table 2 View TABLE 2 ). These important diagnostic characters are in line with key taxonomic traits of R. palustris given in different Floras (Boissier 1867, Rechinger 1964, Cullen 1967, Freeman & Reveal 2005). In addition, R. palustris shares some characters such as stem indumentum, shape of basal and cauline leaves, occurrence of articulate pedicels and teeth and tubercles on all valves, as well as bisexual flowers and trigonous nuts with two species, R. dentatus and R. maritimus , but it remarkably differs from them based on the main characteristics listed in Table 2 View TABLE 2 . In the morphological comparison, habit, position of branched stem, size of basal leaves and petioles, shape of leaf base, articulation type of pedicels, number and length of teeth, as well as size and color of nuts are the most distinguishing characters separating R. palustris from R. dentatus . It also differs from R. maritimus in the shape of leaf base, length of basal petioles, color of mature inflorescences, valves size, number of teeth, as well as size of nuts (see Table 2 View TABLE 2 ). Most of these characters are also taxonomically important in the whole genus (Boissier 1867, Rechinger 1964, Cullen 1967, Rechinger 1968, Lozinskaya 1970, Mozaffarian 1994, Qaiser 2001, Li et al. 2003, Freeman & Reveal 2005, Yasmin 2009, Uddin et al. 2014, Verloove et al. 2022).
Considering various environmental and genetic factors and the related effects on the morphological diversity and high hybridization in the genus Rumex , the identification and delimitation of the taxa still remain unresolved in Iran. Due to this taxonomical complexity and a broad distributional range of Rumex species in Iran, more effort is needed to delimit the interspecific relationships of the genus. Herein, R. palustris as a new record of the genus has an ambiguous taxonomic border because of its high morphological similarities to two closely-related species, R. dentatus and R. maritimus . In addition, it was firstly reported based on specimens misidentified as R. pulcher Linnaeus (1753: 336) and R. maritimus ( Freeman & Reveal 2005) , confirming its taxonomic complexity. The detailed taxonomical studies, as well as ecological and anatomical examinations are here presented to facilitate delimitation and determination of the new record.
Distribution and ecological notes:— The main distribution realm of Rumex palustris stretches from west and southwest of Asia ( Turkey, Syria, Lebanon, Israel, Jordan, and North Caucasus) to over Europe ( Ukraine, Moldova, Romania, Greece, Belarus, Poland, Slovakia, Hungary, Norway, Sweden, Finland, Denmark, France, Germany, Belgium, Netherlands, Switzerland, Spain, Italy, …) and northwest Africa ( Algeria and Morocco), although it was seen in southwestern regions of North America and also southeastern South Africa as an alien ( Fig. 3 View FIGURE 3 ) (Boissier 1867, Rechinger 1964, Cullen 1967, Freeman & Reveal 2005, Euro+Med 2023, EPPO 2023, COL 2023, GBIF 2023, Portal to the Flora of Italy 2022). Considering distribution range of R. palustris in the southwest Asia, western Turkey is the nearest location to our report in the north Iran ( Cullen 1967, Euro+Med 2023, EPPO 2023). Based on the above distributional pattern, this new record is not far from its native distribution and we consider this species in Iran as native. Ecologically, R. palustris is a cosmopolitan hygrophytic/wetland species known as ‘Marsh Dock’ and grows in shores, ballast grounds, wet meadows, marshes, wet ruderal and riversides across the Europe and America ( Rechinger 1964, Freeman & Reveal 2005). The habitat requirement of the collected samples along the south Caspian shores is similar with general habitat features reported elsewhere.
Material examined: — Iran, Mazandaran Province, Ramsar, ca. 2 km W Moallem boulevard (Casino Blvd.) ( Fig. 3 View FIGURE 3 ), on wet coastal dune slacks, 36°55’36.12” N, 50°40’11.69” E, - 26 m, 10 May 2022, Naqinezhad 9115 ( HUMZ, TARI). GoogleMaps
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rumex palustris Smith (1800: 394)
| Khorasani, Mina & Naqinezhad, Alireza 2023 |
Rumex laxiflorus
| Saint-Lager, J. B. 1889: ) |
Steinmannia flavovirens
| Opiz, F. M. 1852: ) |
Rumex uliginosus
| Arcangeli, G. 1882: ) |
| Gussone, G. 1826: ) |
Lapathum palustre (Sm.)
| Gray, S. F. 1821: ) |
Rumex palustris
| Smith, J. E. 1800: ) |
Rumex limosus
| Formanek, E. 1886: ) |
| Celakovsky, L. 1871: ) |
| Renault, P. A. 1804: ) |
| Thuillier, J. L. 1799: ) |