Cranidiini Günther, 1953
|
publication ID |
https://doi.org/10.11646/zootaxa.4128.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B4D2CD84-8994-4CEF-B647-3539C16B6502 |
|
DOI |
https://doi.org/10.5281/zenodo.6084904 |
|
persistent identifier |
https://treatment.plazi.org/id/387F3068-D331-FF97-FF27-EBDB2707182E |
|
treatment provided by |
Plazi (2016-06-27 12:18:21, last updated 2025-02-18 18:32:58) |
|
scientific name |
Cranidiini Günther, 1953 |
| status |
|
4.2.3. Tribe Cranidiini Günther, 1953 View in CoL
( Figs. 13–19 View FIGURES 13 – 19 , 49 View FIGURES 45 – 51 )
Type-genus: Cranidium Westwood, 1843: 49 .
Cranidiini Günther, 1953: 557 View in CoL .
Brock, 1998b: 26.
Clark-Sellick, 1998: 221.
Zompro, 2004: 135 (in part).
Otte & Brock, 2005: 32 (in part).
Hennemann, Conle & Delfosse, 2007: 358 (in part). Bacteriinae Kirby, 1904a: 348 (in part).
Craspedoniini Bradley & Galil, 1977: 187. [Unnecessary replacement name] Phibalosomini View in CoL (Sectio V: Phibalosomata) Redtenbacher, 1908: 399 (in part).
Description: ♀♀/♂♂ ( Figs. 9–10 View FIGURES 6 – 12 ). Medium-sized to large (body lengths: ♀♀ incl. subgenital plate 107.0–161.0 mm; ♂♂ 78.0–115.0 mm), Cladomorphinae with remarkable sexual dimorphism. Body surface shiny, ♀♀ plain bright green. ♂♂ with short tegmina and well developed alae (48.2–53.2 mm), ♀♀ apterous. ♂♂ elongate and moderately slender with body cylindrical. ♀♀ considerably larger and much broader than ♂♂, body strongly dorsoventrally flattened with meso-, metathorax and abdomen strongly laterally dilated (maximum body width 28.0–34.0 mm); body rhombic in cross-section. Head longer than wide, strongly globose and convex; vertex smooth. No ocelli. A gula is present. Antennae very long, slender and filiform, longer than head, thorax and median segment combined and consisting of>80 antennomeres. Pronotum distinctly narrower than head and mesonotum. Mesothorax of ♂♂ elongate and slender, 2.5x longer than head and pronotum combined, minutely granulose; mesosternum with a fine longitudinal median carina. Mesothorax of ♀♀ broadly and semi-circularly expanded, 1.5x longer than wide. Mesonotum strongly convex dorsally and armed with a cluster of blunt tubercles in the raised anterior portion; lateral margins expanded, lamellate and bluntly dentate. Mesopleurae strongly dilated, forming a longitudinal lamella with the outer margin dentate. Mesosternum strongly tectiform and with a prominent and broad, tuberculose longitudinal median keel; lateral margins slightly expanded and with a longitudinal row of tubercles ( Fig. 15 View FIGURES 13 – 19 ). Median segment distinctly longer (♂♂) or roughly equal in length to metanotum (♀♀). Abdominal segments II–VI of ♂♂ parallel-sided and distinctly longer than wide. In ♀♀ tergites and sternites II–VI with lateral margins strongly expanded and lamellate; all transverse. Praeopercular organ of ♀♀ represented by a small spiniform projection on sternum VII ( Fig. 16 View FIGURES 13 – 19 ). Epiproct and cerci of both sexes very small and considerably shorter than anal segment. Gonapophyses VIII of ♀♀ elongated, up-curving and projecting well beyond posterior margin of anal segment ( Fig. 16 View FIGURES 13 – 19 ). Gonoplacs developed ( Fig. 16 View FIGURES 13 – 19 ). Vomer of ♂♂ produced, well sclerotized and elongate-triangular. Phallus totally covered with minute black denticles. Poculum very bulgy, strongly convex and extending ventrally by more than 2x the body width ( Fig. 17 View FIGURES 13 – 19 ). Subgenital plate of ♀♀ distinctly keeled longitudinally, boat-shaped, tapered towards a narrow ± acute apex and projecting considerably over anal segment ( Fig. 16 View FIGURES 13 – 19 ). All legs elongate and slender (♂♂) to moderately robust (♀♀), all distinctly carinate but entirely unarmed. Femora trapezoidal, tibiae almost triangular in cross-section with dorsal carinae strongly approaching each other. Profemora compressed and curved basally with posterodorsal carina reduced and considerably lower than anterior carina; medioventral carina distinct and roughly midways on ventral surface of femur. Medioventral carina of meso- and metafemora very faint. Tarsi simple. Basitarsi at least as long as following three tarsomeres combined and carinate dorsally with the two dorsal carinae melted with another.
Eggs ( Figs. 18–19 View FIGURES 13 – 19 , 49 View FIGURES 45 – 51 ): Medium-sized, strongly globose and almost spherical in lateral aspect; slightly oval in cross-section. Polar-area with a wide depression. Operculum elliptical, flat and with a convex, net-like hollow expansion which covers the complete disc. Micropylar plate covering about half of capsule-length, broadly ovoid with a raised outer margin and convex central region. Internal micropylar plate open and with a narrowing of the posteromedial gap before it widens into the notch. Median line indistinct, short, very well separated from the plate and distinctly displaced towards the polar-area ( Fig. 49 View FIGURES 45 – 51 ).
Differentiation ( Table 1 View TABLE 1 ): Several features such as the presence of a gula, filiform antennae, the elongate, filiform gonapophyses VIII and presence of gonoplacs in ♀♀, specialized poculum of ♂♂ and presence of a median line in the eggs prove close relation to the South American Cladomorphini (→ 4.2.2). Cranidiini however clearly differs from Cladomorphini by: the shiny body surface; green general colouration; entirely unarmed legs; indistinct medioventral carina of the profemora which is roughly central on the ventral surface of the profemur; roughly trapezoidal cross-section of the profemora and very faint medioventral carina of the meso- and metafemora of both sexes. Furthermore, ♀♀ readily differ by: the strongly flattened and laterally dilated, leaf-like body; convex mesonotum and strongly longitudinally tectiform mesosternum ( Fig. 15 View FIGURES 13 – 19 ). Males are also well distinguished by the strongly enlarged and very bulgy poculum which ventrally extends by more than 2x the body diametre, as well as the specialized phallus which is totally covered with minute black denticles. The eggs differ from those of Cladomorphini by having the micropylar plate oval and relatively shorter (<½ of the dorsal capsule surface) and the posteromedian notch of the plate with a narrowing before it widens into the gap ( Fig. 49 View FIGURES 45 – 51 ).
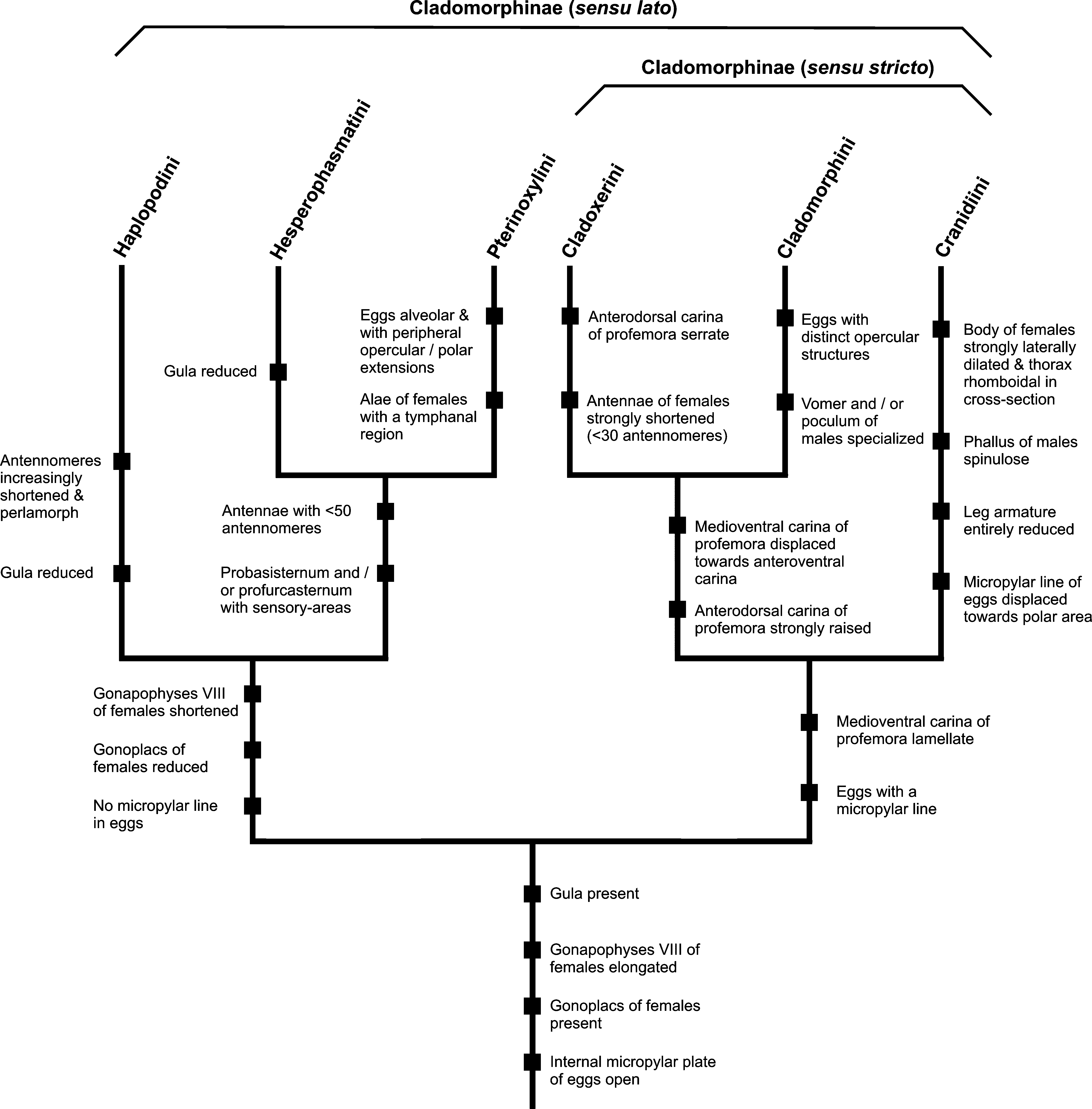
The completely reduced leg armature and tectiform mesosternum of both sexes, the prominently dilated mesothorax and abdomen of ♀♀ as well as the prominently enlarged poculum and spinulose phallus of ♂♂ appear to be autapomorphies of Cranidiini , which suggest the tribe might be the sister-group of Cladomorphini + Cladoxerini ( Fig. 409 View FIGURES 409 ) and at once distinguish it from these two tribes. The elongated gonapophyses VIII and presence of gonoplacs in ♀♀ as well as the developed gula are shared with Cladomorphini and Cladoxerini .
Because of the erroneous and confusing treatment of Cranidiini by Zompro (2004: 135) a detailed comparison and differentiation of Cranidiini and Haplopodini is presented in Table 1 View TABLE 1 , which emphasizes the striking differences between these two tribes and confirms the separate status of Haplopodini as well as the monotypy of Cranidiini .
Comments: When Günther (1953: 557) established the tribe Cranidiini , Cranidium Westwood, 1843 was the only genus contained. A comprehensive treatment and re-description of the type-genus was presented by Hennemann, Conle & Delfosse (2007). Brock (1998b) described a second Jamaican genus in the tribe, Paracranidium . This however, is here shown to be not closely related and to take on a rather isolated position within the re-established tribe Haplopodini Günther, 1953 (→ 5.6), hence is removed from Cranidiini . Another South American genus, Aplopocranidium , was added by Zompro (2004: 135) but is also not closely related to Cranidium and was transferred to Cladomorphini by Hennemann & Conle (2010: 104) who provided a comprehensive treatment of Aplopocranidium including a description of the previously unknown ♂♂.
Zompro (2004: 135) removed the genera Aploploides Rehn & Hebard, 1938 , Haplopus Burmeister, 1838 and Diapherodes Gray, 1835 from Hesperophasmatini Bradley & Galil, 1977 and transferred them to Cranidiini . It is surprising that Zompro (2004: 135) argued the supposed close affinity of these three Antillean genera to Cranidiini exclusively on the fact that they have the same “smooth and shining body” and mentioned this as the only feature that in his opinion characterizes Cranidiini within Cladomorphinae . This act however is very arbitrary, since a wide range of very striking and fundamental morphological differences are present between Cranidiini and the mentioned three genera (→ Table 1 View TABLE 1 ). Nor does this “feature” hold true for all taxa that Zompro transferred to Cranidiini , e.g. ♀♀ of Haplopus -species and ♂♂ of certain Diapherodes -species have a rather dull and ± sculptured body surface. As can be seen in Table 1 View TABLE 1 Aploploides , Haplopus and Diapherodes are not at all closely related to Cranidium and here shown to form a very well separated clade, defined as the supposedly monophyletic tribe Haplopodini rev. stat. (→ 5.). Consequently, these three genera are here removed and Cranidiini is a monotypical and very well separated tribe within the subfamily Cladomorphinae .
Clark-Sellick (1998: 221, fig. 32a, b) and Hennemann, Conle & Delfosse (2007: 360) stated the egg of Cranidiini to lack a median line, although the latter authors showed there to be a median line in the illustration of the internal plate (2007: 365, fig. 12). Subsequent examination of more examples has shown a short and rather indistinct median line to be present. However, it is rather short and very well separated from the micropylar plate, being displaced towards the polar-area by more than its own length.
A captive reared ♀ of C. gibbosum at hand from the first author's collection (coll. FH, No. 0470-14) represents the new length record for this species, measuring 161.0 mm including the subgenital plate.
Distribution: Restricted to Northeastern South America and so far recorded from Northern Brazil, French Guiana and Suriname ( Hennemann, Conle & Delfosse, 2007: 366).
Genus included:
1. Cranidium Westwood, 1843: 49 . Type-species: Diapherodes (Cranidium) serricollis Westwood, 1843: 49 , pl. 6: 1 (= Diapherodes gibbosa Burmeister, 1838 ), by subsequent designation of Bradley & Galil, 1977: 187. = Phasmilliger Carrera, 1960: 100 . Type-species: Diapherodes (Cranidium) serricollis Westwood, 1843: 49 , by indication. [Unnecessary replacement name, hence a junior objective synonym]
Hennemann, F. H., Conle, O. V. & Delfosse, E. (2007) Studies on Neotropical Phasmatodea VI, The genus Cranidium Westwood, 1843 (Phasmatodea, Phasmatidae, Cladomorphinae). Bulletin de la Societe entomologique de France, 112 (3), 357 - 368.
Bradley, J. C. & Galil, B. S. (1977) The taxonomic arrangement of the Phasmatodea with keys to the subfamilies and tribes. Proceedings of the Entomological Society of Washington, 79 (2), 176 - 208.
Brock, P. D. (1998 b) Description of a new genus for a Jamaican stick-insect. Phasmid Studies, 7 (1), 26 - 29.
Burmeister, H. (1838) Handbuch der Entomologie. II. G. Reimer, Berlin, pp. 553 - 589.
Carrera, M. (1960) Insecta amapaensia Diapherodes gibbosa Burmeister, 1839, tipo de um novo genero de Phasmida. Papeis avulsos de Departamento de Zoologica Secretaria da Agricultura, Sao Paulo, 14, 99 - 104.
Gray, G. R. (1835) Synopsis of the species of insects belonging to the family of Phasmidae. Longman, Rees, Orme, Brown, Green and Longman, London, 48 pp.
Gunther, K. (1953) Uber die taxonomische Gliederung und die geographische Verbreitung der Insektenordnung der Phasmatodea. Beitrage zur Entomologie, Berlin, 3, 541 - 563.
Hennemann, F. H. & Conle, O. V. (2010) Studies on Neotropical Phasmatodea X: redescriptions of Aplopocranidium Zompro, 2004 and Jeremia Redtenbacher, 1908, with a survey of the tribe Cladomorphini Brunner v. Wattenwyl, 1893 and keys to the genera (Insecta: Phasmatodea: Anareolatae : Cladomorphinae). Journal of Orthoptera Research, 19 (1), 101 - 113. http: // dx. doi. org / 10.1665 / 034.019.0116
Kirby, W. F. (1904 a) A Synonymic Catalogue of Orthoptera, Vol. 1. British Museum, London.
Otte, D. & Brock, P. D. (2005) Phasmid Species File. Catalog of Stick and Leaf Insects of the World. The Insect Diversity Association and the Academy of Natural Sciences, Philadelphia, 414 pp. [CafePress. com]
Redtenbacher, J. (1908) Die Insektenfamilie der Phasmiden. III. Phasmidae Anareolatae (Phibalosomini, Acrophyllini, Necrosciini). Verlag Wilhelm Engelmann, Leipzig, pp. 341 - 589, pls. 16 - 27.
Rehn, J. A. G. & Hebard, M. (1938) New genera and species of West Indian Mantidae and Phasmidae (Orthoptera). Transactions of the American Entomological Society, 64, 33 - 55, pls. 3 - 4.
Westwood, J. O. (1843) Arcana Entomologica, or illustrations of new, rare, and interesting exotic Insects. II. William Smith, London, 2 pp. + 1 pl. [pp. 49 - 50, pl. 61]
Zompro, O. (2004) A key to the stick-insect genera of the Areolatae of the New World, with description of several new taxa. Studies on Neotropical Fauna and Environment, 39 (2), 133 - 144. http: // dx. doi. org / 10.1080 / 01650520412331333783
FIGURES 409. Possible relationships within the subfamily Cladomorphinae sensu lato. The tree shown above is merely meant to visualise the possible relationships here suggested. It does not restrict to defining apomorphies for each clade or taxon, but also uses key-features that are helpful for distinction. Hence, it should not be interpreted as a strict phylogenetic cladogramm.
FIGURES 13 – 19. Tribe Cranidiini Günther, 1953. 13. Cranidium gibbosum (Burmeister, 1838) ♀: captive reared from French Guiana [coll. FH, No. 0470 - 11]; 14. Cranidium gibbosum ♂: captive reared from French Guiana [coll. FH, No. 0470 - 9]; 15. Cranidium gibbosum: mesosternum of ♀ [coll. FH, No. 0470 - 1]; 16. Cranidium gibbosum: apex of ♀ abdomen (lateral view) [coll. FH, No. 0470 - 17]; 17. Cranidium gibbosum: apex of ♂ abdomen (lateral view) [coll. FH, No. 0470 - 19]; 18. Cranidium gibbosum: egg (dorsal view) [coll. FH, No. 0470 - E]; 19. Cranidium gibbosum: egg (lateral view) [coll. FH, No. 0470 - E]. Gpl = Gonoplacs, Gap 8 = Gonapophyses VIII, Gap 9 = Gonapophyses IX, PrOrg = Praeopercular organ, Po = Poculum, Cer = Cerci, Vo = Vomer, ML = Micropylar line.
FIGURES 45 – 51. Internal micropylar plates of Cladomorphinae and Xerosomatinae. 45. Tribe Haplopodini: Haplopus micropterus (St. Fargeau & Audinet-Serville, 1825); 46. Tribe Pterinoxylini n. trib.: Pterinoxylus crassus Kirby, 1889; 47. Tribe Hesperophasmatini: Lamponius guerini (Saussure, 1868); 48. Tribe Hesperophasmatini: Rhynchacris ornata Redtenbacher, 1908; 49. Tribe Cranidiini: Cranidium gibbosum (Burmeister, 1838); 50. Tribe Cladomorphini: Cladomorpus rubus (Saussure, 1861); 51. Pseudophasmatidae: Xerosomatinae: Xerosomatini: Creoxylus spinosus (Fabricius, 1793).
FIGURES 6 – 12. Tribe Cladomorphini Brunner v. Wattenwyl, 1893. 6. Cladomorphus phyllinus Gray, 1835 ♀: Brazil, Espírito Santo [coll. FH, No. 0184 - 1]; 7. Cladomorphus phyllinus ♂: Brazil, Espírito Santo [coll. FH, No. 0184 - 2]; 8. Cladomorphus phyllinus: apex of ♀ abdomen (lateral view) [coll. FH, No. 0184 - 1]; 9. Cladomorphus phyllinus: apex of ♀ abdomen (dorsolateral view) [coll. FH, No. 0184 - 1]; 10. Cladomorphus phyllinus: apex of ♂ abdomen: Brazil [coll. FH, No. 0184 - 2]; 11. Cladomorphus phyllinus: egg from Brazil, Sao Paulo (dorsal view) [coll. FH, No. 0184 - E 2]; 12. Cladomorphus phyllinus: egg from Brazil, Sao Paulo (lateral view) [coll. FH, No. 0184 - E 2]. Ep = Epiproct, Cer = Cerci, Pa = Paraproct, Gpl = Gonoplacs, Gap 8 = Gonapophyses VIII, PrOrg = Praeopercular organ, Vo = Vomer, Po = Poculum, ML = Micropylar line.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cranidiini Günther, 1953
| Frank H. Hennemann, Oskar V. Conle & Daniel E. Perez-Gelabert 2016 |
Cranidiini Günther, 1953 : 557
| Gunther 1953: 557 |
1 (by plazi, 2016-06-27 12:18:21)
2 (by ImsDioSync, 2016-06-27 13:15:47)
3 (by ImsDioSync, 2016-06-27 14:03:37)
4 (by ImsDioSync, 2017-01-25 13:16:42)
5 (by ImsDioSync, 2017-02-08 02:47:47)
6 (by ExternalLinkService, 2019-09-26 09:02:54)
7 (by ExternalLinkService, 2022-01-30 00:12:34)
8 (by ExternalLinkService, 2022-02-15 03:00:02)
9 (by plazi, 2023-10-30 03:53:11)
10 (by ExternalLinkService, 2023-10-30 11:08:37)
11 (by guilherme, 2025-02-18 18:14:16)