Mustela sibirica Pallas, 1773
|
publication ID |
https://doi.org/ 10.1093/mspecies/sey013 |
|
publication LSID |
lsid:zoobank.org:pub:6625EDBE-8629-46FB-A3EB-29300D954CFF |
|
DOI |
https://doi.org/10.5281/zenodo.4593310 |
|
persistent identifier |
https://treatment.plazi.org/id/35613061-0B48-A609-6C44-86B92BDC11A5 |
|
treatment provided by |
Felipe |
|
scientific name |
Mustela sibirica Pallas, 1773 |
| status |
|
Mustela sibirica Pallas, 1773 View in CoL
Siberian Weasel
Mustela sibirica: Pallas, 1773:701 View in CoL . Type locality: “Sibiriae montanis, sylvis densissimis;” restricted to “Vorposten Tigerazkoi, near Usstkomengorsk, W. Altai” (Oskemen, Kazakhstan, 49.9833° N, 82.6167° E) by Pocock 1941. First use of current name combination.
Viverra sibirica: Shaw, 1800:431 . Name combination.
P [utorius] sibericus: Griffith, 1827:122. Name combination.
Mustela [Putorius View in CoL ] subhemachalana Hodgson, 1837:563. Type locality “ Nepal.”
M [ustela] canigula Hodgson, 1842:280. Type locality “ Tibet.”
[ Mustela View in CoL ] humeralis Blyth, 1842:99. Type locality “ Sikkim.”
Mustela hodgsoni Gray, 1843:118 View in CoL . Type locality “ India, Himalaya.”
Mustela horsfieldii Gray, 1843:118 View in CoL . Type locality “ Bhutan, India.”
Vison sibirica: Gray, 1865:117 . Name combination.
Putorius fontanierii Milne Edwards, 1871:205 View in CoL . Type locality “la Chine;” description based on a specimen obtained by M. Fontanier in China.
Putorius davidianus Milne Edwards, 1874:343 View in CoL . Type locality “Kiang-si, [Moupin, Tibet].”
Putorius moupinensis Milne Edwards, 1874:347 View in CoL . Type locality “Moupin in Szechwan.”
Putorius sibiricus View in CoL miles Barrett-Hamilton, 1904:391. Type locality “Dauria, Eastern Siberia.”
Putorius sibiricus noctis Barrett-Hamilton, 1904:391 View in CoL . Type locality “San-yen-tze, China.”
Lutreola stegmanni Matschie, 1907:150 View in CoL . Type locality “Tsingtao, Shantung.”
Lutreola quelpartis Thomas, 1908:53 View in CoL . Type locality “Island of Quelpart, S. of Korea.”
Lutreola major Hilzheimer, 1910:310 View in CoL . Type locality “ Tibet.”
Lutreola tafeli Hilzheimer, 1910:310 View in CoL . Type locality “ Tibet.”
Kolonokus sibiricus australis Satunin, 1911:266 View in CoL . Type locality “ Tyumen.”
M [ustela] manchurica Brass, 1911:262. Type locality “Manchuria.”
Mustela [Lutreola View in CoL ] taivana Thomas, 1913:91. Type locality “ Formosa.”
Kolonocus sibirica sibirica Satunin, 1914:124 . Name combination.
Mustela hamptoni Thomas, 1921:500 View in CoL . Type locality “Imaw Bum.”
Kolonocus sibiricus coreanus Domaniewski, 1926:55 View in CoL . Type locality “ Seoul.”
Kolonocus sibiricus peninsulae Kishida, 1931:380 View in CoL . Type locality unknown.
Mustela [Kolonocus] sibirica View in CoL charbinensis Lowkashkin, 1934:49. Type locality “Manchuria.”
CONTEXT AND CONTENT. Order Carnivora View in CoL , family Mustelidae View in CoL , subfamily Mustelinae View in CoL . Twelve subspecies are currently recognized, 11 listed by Wozencraft (2005) and M. sibirica taivana proposed by Suzuki et al. (2013). A revision of subspecies taxonomy, however, is needed as up to 22 subspecies have been proposed (Larivière and Jennings 2009).
M. s. canigula Hodgson, 1842:280. See above.
M. s. charbinensis Lowkashkin, 1934:49. See above.
M. s. coreanus Domaniewski, 1926:55. See above; peninsulae Kishida, 1931:380 is a synonym.
M. s. davidiana Milne Edwards, 1874:343. See above; noctis Barrett-Hamilton, 1904:391 is a synonym.
M. s. fontanierii Milne Edwards, 1874:205 View in CoL . See above; stegmanni Matschie, 1907:150 View in CoL is a synonym.
M. s. hodgsoni Gray, 1843:118 View in CoL . See above.
M. s. manchurica Brass, 1911:262. See above.
M. s. moupinensis Milne Edwards, 1874:347 View in CoL . See above; hamptoni Thomas, 1921:500 View in CoL , major Hilzheimer, 1910:310 View in CoL , and tafeli Hilzheimer, 1910:310 View in CoL are synonyms.
M. s. quelpartis Thomas 1908:53 View in CoL . See above.
M. s. sibirica Pallas, 1773:701 . See above; australis Satunin, 1911: 280 miles Barrett-Hamilton, 1904:391 are synonyms.
M. s. subhemachalana Hodgson, 1837:563. See above; humeralis Blyth, 1842:99 and horsfieldii Gray, 1843:118 View in CoL . are synonyms.
M. s. taivana Thomas, 1913:91. See above. NOMENCLATURAL NOTES. Mustela sibirica has been previously placed in the genus Viverra ( Shaw 1800) , genus Putorius ( Griffith 1827) , genus Vison ( Gray 1865) , genus Lutreola ( Matschie 1907) , genus Kolonokus ( Satunin 1911) , and genus Kolonocus ( Satunin 1914) . In addition, M. sibirica has also been placed under the subgenus Lutreola ( Youngman 1982) and later in the subgenus Kolonokus ( Abramov 1999) . Other vernacular names include the kolonok and kolinsky ( Novikov 1962).
DIAGNOSIS
Mustela sibirica occurs sympatrically with a variety of mustelids including ferret-badgers, martens, otters, and weasels and stoats (mustelines). Mustelines like M. sibirica can be distinguished from many other mustelids by their small sizes and elongated bodies. In its natural ranges in Asia, M. sibirica can be distinguished from most sympatric mustelines—mountain weasel M. altaica , ermine M. erminea , yellow-bellied weasel M. kathiah , least weasel M. nivalis , and the introduced American mink Neovison vison —by the presence of a black mask on its face that surrounds its eyes, a white muzzle and chin, and a nearly completely monotone yellowish-brown coat in winter ( Fig. 1 View Fig ). The sympatric Steppe polecat M. eversmanii also exhibits a dark mask that surrounds its eyes but the mask extends farther across its face toward the cheeks. In addition, M. eversmanii exhibits a white band between the ears and eyes that crosses its head from cheek to cheek ( Heptner et al. 2001; Larivière and Jennings 2009). Other characteristics that distinguish M. sibirica from M. eversmanii are body size ( M. eversmanii can attain a body mass twice that of M. sibirica ) and coat color ( M. eversmanii exhibits a coat with a combination of yellowish-white and dark brown color, whereas M. sibirica exhibits a nearly completely monotone yellowish-brown coat in winter and a dark brown coat in summer—Heptner et al. 2001; Larivière and Jennings 2009). On the Japanese islands of Honshu, Shikoku, and Kyushu, introduced populations of M. sibirica occur sympatrically with the Japanese weasel M. itatsi . Characteristics that distinguish M. sibirica from M. itatsi include body size ( M. sibirica is larger than M. itatsi ), the ratio of tail (T) length to body (HB) length (T/HB ratio is> 50% in M. sibirica , whereas the T/HB ratio is <40% in M. itatsi ), and coat color ( M. sibirica exhibits a lighter brown coat than M. itatsi in winter—Masuda et al. 2012).
GENERAL CHARACTERS
Mustela sibirica is sexually dimorphic and males are almost twice as heavy as females (Larivière and Jennings 2009). Body weight is 650–820 g for males and 360–430 g for females ( Hunter 2011). Body length is 28–39 cm for males and 25–30.5 cm for females, and tail length is 15.5–21 cm for males and 13.3–16.4 cm for females ( Hunter 2011).
Like other mustelines, M. sibirica has a long, slender body with short limbs. The summer pelage is characterized by short, coarse hair with a dark brown color almost completely covering the entire body and tail; the winter pelage is denser and pale, yellowish-brown in color ( Heptner et al. 2001; Larivière and Jennings 2009). The face exhibits a dark mask around in front of the eyes with a white muzzle and chin ( Hunter 2011). Females have 4 pairs of mammae ( Pocock 1941).
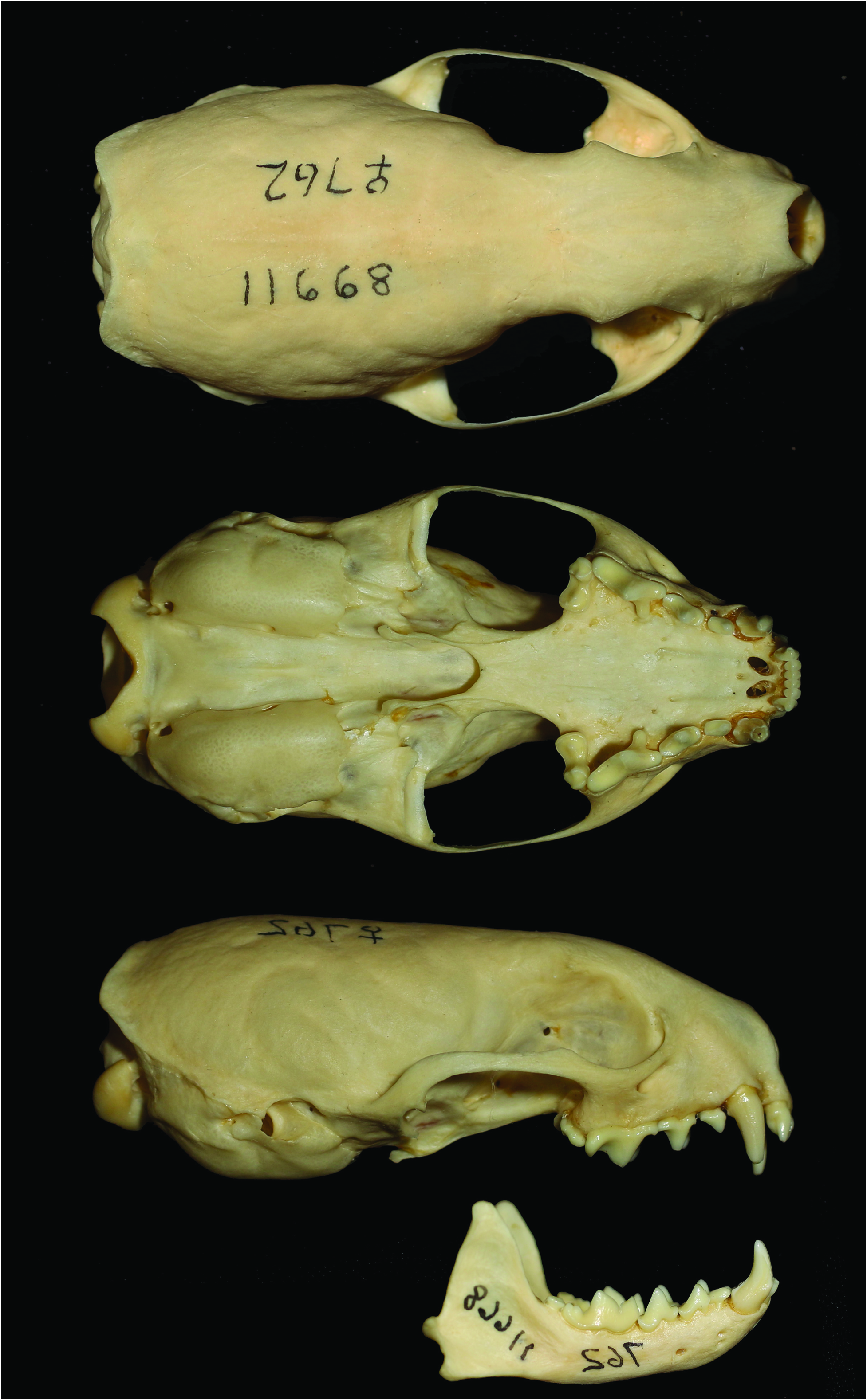
The skull is characterized as long and narrow ( Heptner et al. 2001; Fig. 2 View Fig ). Mean skull measurements (mm, with ranges in parenthesis) for adult male and female M. s. sibirica in Russia, respectively, were: condylobasal length, 61.7 (58.0–63.5), 52.8 (49.8–56.3); zygomatic breadth, 32.2 (28.7–35.7), 27.8 (26.4– 29.6); interorbital width, 11.7 (11.7–13.2), 11.0 (10.5–12.2); mastoid width, 27.5 (26.8–28.7), 24.3 (23.0–26.1— Heptner et al. 2001). The skulls of male M. sibirica are 16.25% larger than the skulls of females (Law and Mehta 2018).
For mustelids in general, the degree of sexual dimorphism in body mass and length can be strongly impacted by the food supply for a cohort during growth, and dimorphism in body size often exceeds that for teeth and jaws (King and Powell 2007). M. sibirica does exhibit sexual dimorphism in craniodental size but little in shape ( Sheng 1987; Abramov and Puzachenko 2009; Suzuki et al. 2011). Discriminant analyses using 45 craniodental linear measurements found the following characters contributed to larger skull size in males compared to females: relatively wide viscerocranium; wide postorbital constriction; a slender, long, and high neurocranium; short and wide auditory bullae; short carnassials; and a long and high mandible ( Suzuki et al. 2011). The degree of sexual size dimorphism varies across the species’ geographic range ( Sheng 1987; Abramov and Puzachenko 2009; Suzuki et al. 2013). Abramov and Puzachenko (2009) found that the subspecies M. s. manchurica of the Far East displays a greater degree of sexual size dimorphism than M. s. sibirica of western and central Siberia. In China, populations occurring in the river plains near the Yangtze and Huai rivers are generally larger and exhibit greater sexual size dimorphism than conspecifics occurring in forest habitats of the Changbai Mountains ( Sheng 1987). In addition, male and female individuals of insular populations exhibit smaller skull sizes; the subspecies M. s. taivana in Taiwan exhibits significantly smaller skulls compared to M. s. davidiana of southeast China ( Suzuki et al. 2013). Similarly, populations of M. s. coreana in Tsushima Island are slightly smaller than populations of conspecifics found in South Korea ( Suzuki et al. 2013).
The baculum is weakly curved; the distal tip is flattened and bent upwards, forming a slight hook ( Heptner et al. 2001; Baryshnikov et al. 2003). Mean measurements (mm, ranges in parenthesis) for adult males and juvenile males, respectively, were: length, 33.9 (32.0–35.8), 32.2 (30.2 –34.2); width of base, 2.15 (0.6–3.7), 1.6 (0.5–2.7); height of base, 3.65 (2.0–5.3), 2.25 (1.5–3.0— Novikov 1962).
DISTRIBUTION
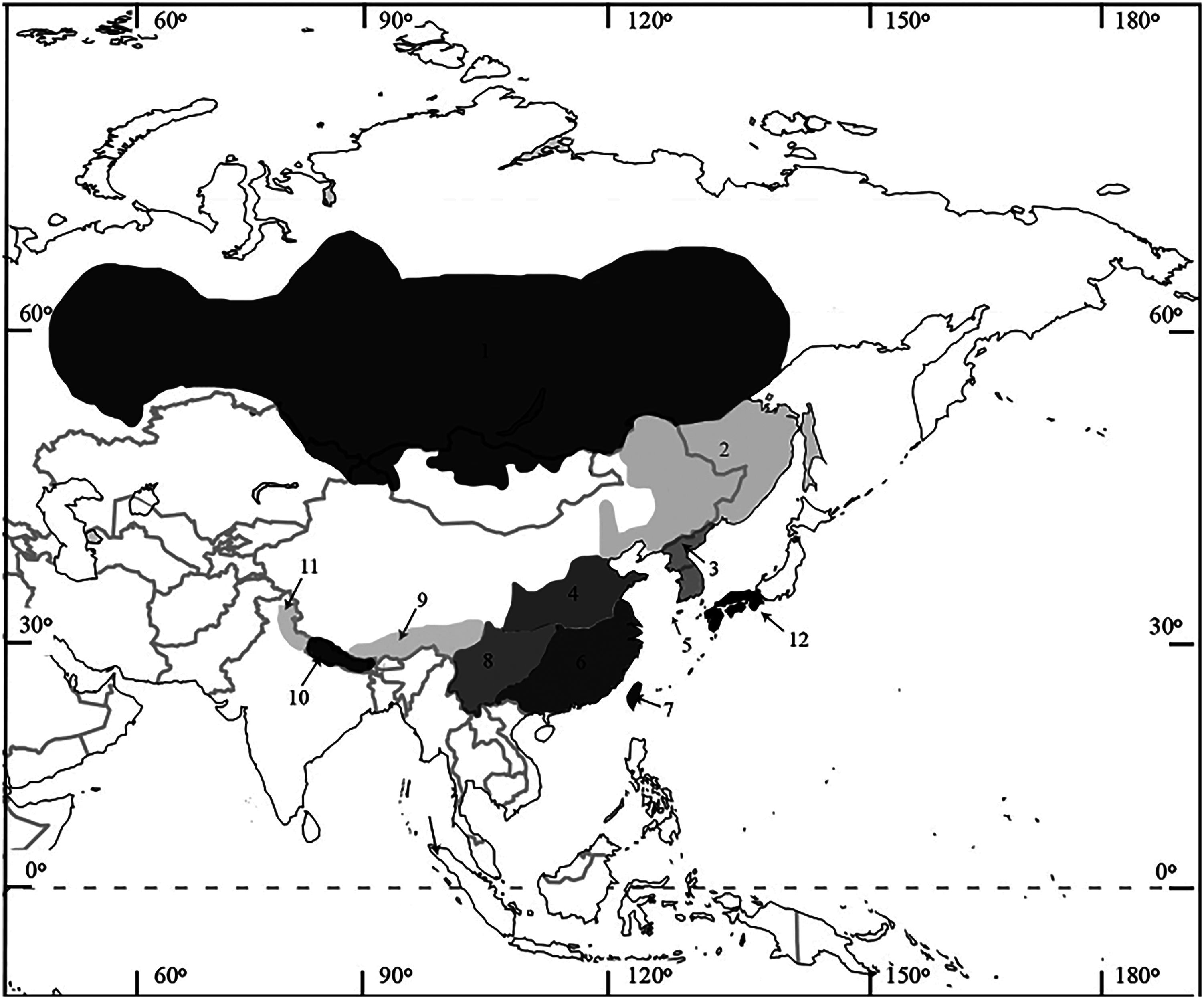
Mustela sibirica is widely distributed across Palearctic Asia, with natural populations ranging from the western base of the Ural Mountains of Siberia to the Far East and south to Taiwan and the Himalayas ( Abramov et al. 2016; Fig. 3 View Fig ). M. s. sibirica occurs in all of Siberia, ranging from the Kostroma Oblast to 63°N in the Ural Mountains and the upper reaches of the Pur River and down south to the northern border of Kazakhstan and the Altai Mountains ( Bakeev 1971; Kassal 2013; Abramov et al. 2016). The range continues eastward through the Zeya Basin and Mongolia and ends at the western parts of northeastern China (Manchuria—Heptner et al. 2001).
Both M. s. charbinensis and M. s. manchurica occur in northeastern China (Manchuria—Heptner et al. 2001); however, the exact ranges are unknown and the validity of these separate subspecies remains untested. M. s. coreana is endemic to the Korean Peninsula and to Tsushima, Japan (Sasaki and Ono 1994). M. s. fontanierii occurs in the northern parts of Anhui, eastern parts of Gansu, southern parts of Hebei, Henan, northern parts of Hubei, northern parts of Jiangsu, southern parts of Nei Mongol, Shaanxi, Shandong, Shanghai, and Shanxi (China— Allen 1929; Smith et al. 2010). M. s. quelpartis is endemic to Jeju Island (formerly Quelpart Island), Japan ( Abramov 2005).
Two subspecies occur at the southeast edge of the species’ geographic range: M. s. davidiana occurs in southeast China (Anhui, Fujian, Guangdong, Guangxi, Guizhou, Hubei, Hunan, Jiangxi, Shaanxi, Sichuan, and Zhejiang) and M. s. taivana is endemic to Taiwan ( Smith et al. 2010; Suzuki et al. 2013). M. s. moupinensis occurs in the Chinese provinces of Gansu, Guizhou, western parts of Hubei, southeastern parts of Qinghai, southern parts of Shaanxi, Sichuan, Yunnan (Ellerman and Morrison-Scott 1951; Smith et al. 2010).
Three subspecies occur around the Himalayas: M. s. canigula occurs in Tibet ( Hodgson 1842; Heptner et al. 2001); M. s. subhemachalana occurs in Nepal to Bhutan (Ellerman and Morrison-Scott 1951); and M. s. hodgsoni occurs in Kashmir and the Western Himalayas from Kam to Garhwal ( Gray 1843; Heptner et al. 2001).
Mustela sibirica was released from fur farms in Hyogo and has since spread to the Japanese islands of Honshu, Shikoku, and Kyushu ( Miyashita 1963; Sasaki et al. 2014). M. s. sibirica was also reintroduced in the Semenov District of Nizhny Novgorod Oblast, Russia in 1937 and in the Dzhetyoguz District of Issyk Kul Province, Kyrgyzstan in 1941 ( Heptner et al. 2001; Long 2003). No fossils are known for M. sibirica .
FORM AND FUNCTION
The dental formula for Mustela sibirica is i 3/3, c 1/1, p 3/3, m 1/2, total 34 (Larivière and Jennings 2009). Comparison of the craniodental morphology using 32 linear measurements found subtle shape differences between 5 populations of M. sibirica (southeast China, Korea, Tsushima, Honshu, and Taiwan— Suzuki et al. 2013). Skulls from insular populations tend to be smaller than continental specimens ( Suzuki et al. 2013).
In northern Russia, the spring molt occurs toward the end of February or the beginning of March; the winter guard hairs shed and the pelage is quickly replaced with summer guard hairs ( Novikov 1962). The autumn molt occurs at the end of August or the beginning of September ( Novikov 1962). The winter guard hairs grow out simultaneously with the loss of summer guard hairs, and the winter pelage is completely grown out by early November ( Novikov 1962). In Heilongjiang, China, autumn molt begins around October–November ( Hua et al. 2010). Increased in hair densities (hairs/mm 2) from summer to winter coats in males and females, respectively, were: 91.82 to 219.33 and 73.83 to 182.35 on the head; 121.93 to 263.98 and 105.99 to 205.50 on the back; 73.89 to 175.12 and 65.91 to 151.26 on the belly; and 80.38 to 183.59 and 73.21 to 180.63 on the tail ( Hua et al. 2010). Increase in hair lengths (mm) from summer to winter coats in males and females, respectively, were: 11.50 to 17.84 and 11.48 to 14.27 on the head; 20.82 to 27.01 and 18.90 to 25.18 on the back; 15.97 to 19.82 and 14.06 to 18.33 on the belly; and 30.36 to 42.61 and 23.59 to 41.63 on the tail ( Hua et al. 2010).
In the Longkou Forest Farm of Tonghe in the Xiaoxingan Mountain, China, mean measurements (ranges in parenthesis) of the winter guard hairs from the mid-backs of 15 adult males and 15 adult females, respectively, were: hair length, 33.50 mm (32.00–36.00 mm), 28.85 mm (25.50–31.50 mm); hair follicle length, 0.37 mm (0.23–0.40 mm), 0.27 mm (0.20–0.34 mm); hair diameter, 126.6 µm (108.4–152 µm), 79.41 µm (98.5– 147.8 µm); and hair root diameter, 26.8 µm (19.7–39.4 µm), 22.5 µm (19.7–29.6 µm—Zhang et al. 2008). Mean measurements (ranges in parenthesis) of winter upper-hairs from the hind-toes of 15 adult males and 15 adult females, respectively, were: hair length, 11.32 mm (9.31–14.28 mm), 10.45 mm (9.10–11.59 mm); hair follicle length, 0.91 mm (0.46–1.33 mm), 0.79 mm (0.11–1.21 mm); hair diameter, 107.7 µm (91.6– 119.2 µm), 101.0 µm (88.7–108.4 µm); and hair root diameter, 86.0 µm (68.0– 109.3 µm), 71.9 µm (59.1–88.7 µm—Zhang et al. 2008).
The anal gland contains 9 sulfur-based volatiles: 2,2-dimethylthietane, (E)-2,4-dimethylthietane, (E)-2,3-dimethylthietane, 2-ethylthietane, (E)-2-ethyl-3-methylthietanes, (Z)-2-ethyl- 3-methylthietanes, 2-propylthietane, 3,3-dimethyl-1,2-dithiacyclopentane, and (Z)-3,4-dimethyl-1,2-dithiacyclopentane; (E)-2,2-dimethylthietane is the most abundant ( Zhang et al. 2002). Volatile abundance differs between the sexes: (E)-2,4- dimethylthietane and (E)-2,3-dimethylthietane are significantly more abundant in females than in males, whereas 3,3-dimethyl- 1,2-dithiacyclopentane is significantly more abundant in males than in females ( Zhang et al. 2002, 2003). 2-Ethylthietane only occurs in females and is undetected in males ( Zhang et al. 2002, 2003). Laboratory experiments reveal that rice-field rats Rattus rattoides exhibit self-anointing behavior when presented filter paper scented with anal-gland secretions of M. sibirica ( Xu et al. 1995) .
ONTOGENY AND REPRODUCTION
Little is known about the reproduction of Mustela sibirica . In Siberia, the breeding season occurs at the beginning of February to the end of March ( Heptner et al. 2001). Captive M. sibirica in Novosibirsk, Russia, however, bred from April to August, with peak breeding activity occurring in late April ( Ternovsky 1977, not seen, cited inAmstislavsky and Ternovskaya 2000:572). This variation in timing may be due to differences in environmental conditions, including those imposed by captivity. Copulation lasts from 27 min to up to 2 h and 40 min (Ternovsky and Ternovskaya 1994). M. sibirica has the shortest gestation period (32–35 days; mean 33.5 days) of all studied mustelids ( Ternovsky 1977, not seen, cited in Amstislavsky and Ternovskaya 2000:572). Liter size ranges 2–12 kits (mean 6.2 kits) ( Ternovsky 1977, not seen, cited in Amstislavsky and Ternovskaya 2000:572). M. sibirica does not exhibit delayed implantation ( Mead 1989).
Young are born blind and almost naked with only sparse white fur ( Heptner et al. 2001). Young open their eyes for the 1st time by 28–30 days, and weaning ends at the end of the 2nd month ( Heptner et al. 2001). Young born in April become independent toward the end of summer, usually by August ( Novikov 1962).
ECOLOGY
Population characteristics. —The range of Mustela sibirica is extensive across Palearctic Asia, with natural populations ranging from west of the Ural Mountains of Siberia to the Far East and south to Taiwan and the Himalayas ( Abramov et al. 2016). Food abundance is hypothesized to determine the population and distribution of M. sibirica , and Siberia and northeast China are believed to contain the highest densities of M. sibirica because of large densities of several rodent species ( Heptner et al. 2001).
Mustela sibirica is a common game species in western Siberia, and records of population censuses are largely based on fur trapping records ( Bakeev 1971). Long-term records reveal great annual and multi-annual fluctuations in population density. Increases in population densities were preceded by large increases in rodent abundance ( Bakeev 1971). The mean total number of M. sibirica trapped during the early to mid-1900s are 25 in the Kostroma Oblast, 437 in the Orenburg Oblast, 422 in the Republic of Tatarstan, 525 in the Sverdlovsk Oblast, and 86 in the Tyumen Oblast ( Bakeev 1971). Since the 1950s, the decline in successful fur trappings suggested that population densities in several regions decreased, which may be attributed to the combination of deforestation and reduction in rodent abundance ( Bakeev 1971). Low fur prices also may reduce number of individuals trapped ( Abramov et al. 2016). In the Sverdlovsk Oblast, Russia, M. sibirica experienced a 39–71% decline in total population abundance from 1987 to 2011 ( Monakhov 2011a).
In Kyushu, Japan, population density of introduced M. sibirica is 4–15 individuals per km ( Sasaki et al. 2014). The mean longevity for wild M. sibirica is calculated to be 2.1 years (Miyagi and Shiraishi 1978).
Space use. — Mustela sibirica is found in a wide variety of habitats including dense primary and secondary deciduous, coniferous, and mixed forests; woodlands; open grasslands; and river valleys ( Heptner et al. 2001; Abramov et al. 2016). M. sibirica prefers regions near lakes and swamps covered with bushes and fallen trees where small rodents are abundant ( Novikov 1962). M. sibirica is well documented at high elevation: 1,400 –1,700 m in the secondary forests of Guandaushi Forest, Taiwan ( Wu 1999); 2,700 –3,700 m in the primary forests of the Tawu Mountains, Taiwan ( Chiang et al. 2012);> 3,000 m in Nepal ( Ghimirey et al. 2014); 1,500 –4,800 m in Bhutan ( Abramov et al. 2016); and up to 5,000 m in China ( Abramov et al. 2016).
Mustela sibirica uses fallen logs, empty stumps, and brushwood piles as shelters and nests ( Heptner et al. 2001). Individuals also inhabit the burrows of their prey, such as voles, mice, and pikas ( Heptner et al. 2001). Near Lake Baikal in Russia, burrows ranged from 0.6 to 4.2 m in length and from 0.2 to 1.3 m in depth, and the nesting chamber located in the middle of the burrow was lined with feathers or fur from prey ( Fetisoff 1936). Each individual usually has 1 primary burrow as well as many secondary refuges across its range, which may extend for several kilometers ( Fetisoff 1936).
Diet. — Mustela sibirica exhibits a mesocarnivorous (50–70% vertebrate prey) to hypercarnivorous (> 70% vertebrate prey) diet that is largely dependent on the habitat and location. Small voles, mice, and pikas constitute the basic diet of M. sibirica in most locations ( Fetisoff 1936; Novikov 1962; Heptner et al. 2001). Larger sized rodents such as chipmunks, invasive muskrats, and other squirrels are also preyed upon ( Heptner et al. 2001). Birds, amphibians, fish, eggs, berries, and nuts are consumed when rodents are not available ( Novikov 1962).
On the Tsushima Islands of Japan, scat analyses (n = 218) reveal that M. sibirica exhibits a mesocarnivorous diet: small mammals (35%, average percentage of relative occurrence), insects (20%), berries and seeds (13%), birds (10%), other plant material (10%), earthworms (7%), and amphibians and reptiles (5%—Tatara and Doi 1994). The Shannon–Weaver’s diversity index (H ′) of the prey items was 1.869 (Tatara and Doi 1994). M.sibirica exhibits seasonal differences in diets. Caterpillars and beetles are common in spring and summer (24.4–31.8%), and earthworms (19.8%) are consumed during the autumn (Tatara and Doi 1994). During winter, tetrapods comprise nearly 80% of the diet, including an increase in bird consumption (24.5%). Small mammals remain the most common prey throughout the year (22.6–48.9%), and a large portion consists of mainly house mice, Mus musculus , and wood mice, the large Japanese field mouse Apodemus speciosus and the small Japanese field mouse Apodemus argenteus (Tatara and Doi 1994) . Surprisingly, plant materials also occur throughout the year (12.8–28.6%—Tatara and Doi 1994).
Scat (n = 115) from the grasslands of Aoshima, Japan, also reveal a mesocarnivorous diet; insects (68.7%, average percentage of absolute occurrence), mammals (48.7%), amphibians (13.0%), fish (12.2%), and reptiles (9.6%) are the dominate prey items (Sasaki and Ono 1994).
In the Guandaushi Forest of Taiwan, scat analyses (n = 157) reveal that arthropods (43.6%, average percentage of relative occurrence), small mammals (26.0%), and earthworms (17.6%) are the dominant prey items in this region ( Wu 1999). The Chinese white-toothed shrew Crocidura kurodai and the lesser Taiwanese shrew Chodsigoa sodalis are the most important mammalian prey, occurring in one-third of all analyzed scats ( Wu 1999). On the other hand, in high elevation alpine grasslands in Taiwan, M. sibirica exhibits a hypercarnivorous diet where small mammals, particular rodents including the Oldfield white-bellied rat Niviventer culturatus , the Taiwan field mouse Apodemus semotus , Kikuchi’s field vole Microtus kikuchii , and Père David’s vole Eothenomys melanogaster (92.0%, average percentage of relative occurrence), are the most dominant prey ( Ma 1990).
Diseases and parasites. —In Hokkaido, Japan, intestinal parasitic worms found in Mustela sibirica include 3 nematodes Capillaria putorii , Strongyloides , and Spiruidea (larva); 1 trematode Echinostoma hortense ; and 1 acanthocephalan Centrorhynchus elongatus (juvenile) (Kamiya and Ishigaki 1972). In addition, the nematode Filaroides martis was found in the lungs (Kamiya and Ishigaki 1972). In the Tohoku region, the intestinal fluke E. hortense was found in 2 M. sibirica individuals ( Sato et al. 1999). Lung fluke infections caused by Paragonimus miyazakii and P. ohirai were found in animals from the Miyazaki Prefecture, Japan ( Ashizawa et al. 1980). Other parasites found in Japanese M. sibirica populations include the trematodes Clonorchis sinensis , Echinostoma trigonocephala , Heterophyes heterophyes , Isthmiophora melis , and Paragonimus westermani ; the tapeworms Sparganum mansoni and Dipylidium caninum ; the nematode Gnathostoma spinigerum ; the roundworm Dioctophyme renale and Trichinella ; and the acanthocephalan Centrorhynchus itatsinis ( Yoshida et al. 1932) .
Intestinal parasitic worms found in M. sibirica in Taiwan include 7 nematodes Filaroides (94.4%, frequency of occurrence from 16 individuals), Ancylostoma (77.4%), Uncinaria (35.5%), Trichuris species 1 (35.5%), Trichuris species 2 (19.3%), Capillaria (6.5%), and Physaloptera (3.2%); 1 trematode Platynosomum (74.1%); and 1 acanthocephalan Macracanthorhynchus (10%— Chen 2003). Two species of ticks were also observed: Ixodes ovatus and Haemaphysalis ( Chen 2003) .
In Hoengseonggun, South Korea, the tapeworm Spirometra erinaceieuropaei was found in 1 M. sibirica individual ( Lee et al. 2013). The nematode Gnathostoma nipponicum has also been found in populations in Jejudo, South Korea ( Woo et al. 2011). Some South Korean populations of M. sibirica have also been identified to carry vector-borne diseases through infections from Ehrlichia and Anaplasma species ( Chae et al. 2003).
Parasites found in M. sibirica in Russia include mites Ixodes persulcatus and Dermacentor caina and the nematodes Agamospirura , Filaroides orientalis, Scriabingulus nasicola ( Romanov 1960; Kontrimavichus and Kazakov 1966; Heptner et al. 2001).
The 1st reported cases of neoplasia in M. sibirica occurred in 2 nonwild individuals in Lower Saxony, Germany game reserve ( Zöller et al. 2008). The male specimen exhibited an interstitial cell tumor in the right testicle, and subsequent necropsy revealed tumor lesions within the abdominal cavity and spleen ( Zöller et al. 2008). The female specimen exhibited a fibrosarcoma on the upper left hind limb; the tumor had developed multiple times after removal of the original tumor ( Zöller et al. 2008). M. sibirica can also be infected by canine distemper virus ( Kameo et al. 2012).
Interspecific interactions. — Mustela sibirica occurs sympatrically with other carnivorans including felids, canids, and other mustelids such as martens, ferret-badgers, weasels, and polecats ( Shaposhnikov 1956; Novikov 1962; Bakeev 1971; Tatara and Doi 1994; Wu 1999; Chiang et al. 2012). Spatial, dietary, and temporal variation in resource use have been suggested to limit competition among these carnivores, but no study to date has truly investigated interspecific interactions between M. sibirica and other carnivorans
Mustela sibirica exhibits great dietary overlap with the yellow-throated marten Martes flavigula chrysospila in Tawu Mountain Nature Reserve, Taiwan, suggesting interspecific competition for food ( Chiang et al. 2012). Despite the similar diets, yellow-throated martens exhibit almost exclusively diurnal activity patterns, whereas M. sibirica is almost exclusively nocturnal, thus suggesting that the 2 mustelids limit competition by avoiding each other temporally ( Chiang et al. 2012).
Similarly, although sympatric M. sibirica and Chinese ferret-badgers Melogale moschata have substantial dietary overlap in the Guandaushi Forests of Taiwan, the relative abundance of prey items for each differed significantly ( Wu 1999). In addition, M. sibirica occur at higher elevations (1,400 –1,700 m) characterized by secondary forest and flat terrain, whereas Chinese ferret-badgers occur at lower elevations (850–1,400 m) characterized by primary forests ( Wu 1999).
In the Tsushima Islands of Japan, M. sibirica occurs sympatrically with 2 other species of carnivores: the Tsushima leopard cat Felis bengalensis euptilura and the Tsushima marten Martes melampus tsuensis (Tatara and Doi 1994) . Scat analyses reveal that the 3 carnivores do not compete for food: martens are the most hypocarnivorous and consumed mainly fruits and berries, whereas leopard cats are the most hypercarnivorous and consumed small mammals and birds. M. sibirica exhibits an intermediate, mesocarnivorous diet (Tatara and Doi 1994).
Mustela sibirica co-occurs with the sable Martes zibellina in the taiga forests of the Altai, the Far East, and eastern Siberia ( Bakeev 1971). Sables generally eat small mammals, birds, and vegetation matter such as nuts and berries ( Monakhov 2011b). However, during periods of poor vegetation growth, sables directly compete with M. sibirica for small rodents ( Shaposhnikov 1956). In this situation sables display agnostic behaviors toward M. sibirica and often drive them into open habitats which results in decreased populations of weasels ( Shaposhnikov 1956; Bakeev 1971). Fur of M. sibirica is occasionally found in sable excrement ( Shaposhnikov 1956).
Mustela sibirica is sympatric to 3 other mustelines in the Baraba steppe of Western Siberia: M. erminea , M. nivalis , and M. eversmanii (Abramov and Puzachenko 2012) . Gut-content analyses found that rodents comprised of 100% of each of these 4 mustelines, with great overlap in the consumption of smaller rodent species such as mice and voles (Ternovsky and Danilov 1965). However, analyses on cranial traits suggest that these 4 mustelines occupy different regions of cranial morphospace and thus may utilize different resources (Abramov and Puzachenko 2012). A more comprehensive study is needed to understand patterns of resource partitioning between these 4 Mustela species. Natural hybridization between M. sibirica and M. eversmanii is observed, resulting in hybrids known as “giant kolonoks” that are much larger than typical M. sibirica individuals ( Heptner et al. 2001).
Invasive populations of M. sibirica in Japan are now sympatric with some populations of M. itatsi , and some researchers have postulated that the 2 weasels compete for resources ( Sasaki et al. 2014). No comprehensive study has tested this hypothesis. Recent distribution studies suggest that M. itatsi occurs in grasslands and plantations and avoids urban areas, whereas M. sibirica is more abundant in locations with greater human activity ( Sasaki et al. 2014). Sasaki et al. (2014) speculates that the presence of M. itatsi prevents range expansion of M. sibirica . M. sibirica primarily occurs in western Japan, and although the distribution is slowly expanding eastward, the range cannot expand past the Aichi Prefecture where M. itatsi is dominant ( Sasaki et al. 2014). Known predators of M. sibirica include foxes and large falconiformes ( Novikov 1962).
HUSBANDRY
Mustela sibirica is rarely held in captivity because of the difficulty in raising them. M. sibirica is currently found in only a few zoos such as the Longleat Safari Park in Britain, the Poznan Zoo in Poland, and the Dresden Zoo in Germany. The Experimental Research Station in Novosibirsk, Russia, is the most successful in breeding M. sibirica (Ternovsky and Ternovskaya 1994) . Captive breeding can result in interspecies hybrids between M. sibirica with M. eversmanii , the European mink M. lutreola , and the European polecat M. putorius (Ternovsky and Ternovskaya 1994) . However, whether these hybrids are reproductively viable is unknown. Individuals of M. s. coreana from South Korea were kept in Japanese fur farms in the late 1920s to early 1930s ( Long 2003; Sasaki 2009). M. sibirica are kept in Chinese fur farms ( European Society of Dog and Animal Welfare 2015).
Mustela sibirica and kohhosiks (hybrids between M. sibirica and M. eversmanii ) are kept as pets in Russia ( Russian Ferret Society 2007). The oldest known captive individual lived for 8 years and 10 months ( Jones 1982).
BEHAVIOR
The behavior of Mustela sibirica is not well studied compared to other Palearctic mustelines. M. sibirica is typically crepuscular or nocturnal ( Heptner et al. 2001; Chiang et al. 2012). Nocturnal activities are believed to limit resource competition with other carnivorans such as yellow-throated martens ( Chiang et al. 2012). M. sibirica is solitary with the exception of females raising young ( Nowak 2005). Males do not assist in raising kits ( Novikov 1962).
Like other mustelines, M. sibirica uses anal glands for scent communication, marking territory, and defense ( Pocock 1941). The presence of sex-specific compounds in the anal glands suggests that chemical secretion could be used to code for information between males and females ( Zhang et al. 2003). Individuals caught in traps exhibit ear-piercing screams and anal-gland secretion ( Pocock 1941).
Both daily movements and seasonal migrations are dependent on fluctuations of prey population ( Heptner et al. 2001). M. sibirica can move up to 8 km in a single night ( Nowak 2005). M. sibirica is reportedly a good swimmer and climber and is able to pursue water voles in lakes and chase squirrels in trees ( Novikov 1962).
GENETICS
Mustela sibirica has a diploid number (2n) of 38 chromosomes and a fundamental number (FN) of 58 ( Kurose et al. 2000). The karyotype consists of 7 pairs of metacentrics, 4 pairs of submetacentrics, 7 pairs of acrocentrics, and 2 sex chromosomes ( Kurose et al. 2000).
Mustela itatsi was once classified as a subspecies of M. sibirica . Mitochondrial DNA data have since validated the separation of the 2 Mustela species with divergence times approximately 1.70–2.40 million years ago (Masuda andYoshida 1994). More recent phylogenetic analyses using mitochondrial and nuclear DNA demonstrate that M. sibirica and M. itatsi are distinct species ( Sato et al. 2012). M. sibirica is sister to a clade comprised of M. lutreola , M. nigripes , M. putorius , and M. eversmanii ( Koepfli et al. 2008; Sato et al. 2012; Law et al. 2018).
Genetic analyses using mitochondria DNA (cytochromeb gene) from 5 native populations—Transbaikalia ( Russia), Ural Mountains ( Russia), Taiwan, South Korea, and Tsushima Island ( Japan)—as well as non-native populations on mainland Japan indicated 4 distinct lineages: 1) a Korean lineage including the introduced Japanese population, 2) a Tsushima lineage, 3) a Russian lineage, and 4) a Taiwanese lineage ( Masuda et al. 2012). A separate study also using the cytochrome- b gene found M. s. coreanus from the Korean Peninsula and M. s. quelpartis from Jejudo are not distinctly different, suggesting that these populations may not be 2 distinct subspecies ( Koh et al. 2012).
Mustela sibirica is sympatric to M. eversmanii in the Baraba steppe of western Siberia, and natural hybridization occurs between these 2 species ( Heptner et al. 2001). In addition, captive breeding can result in interspecies hybrids between M. sibirica with M. eversmanni , M. lutreola , and M. putorius (Ternovsky and Ternovskaya 1994) .
CONSERVATION
Mustela sibirica is listed as “Least Concern” by the International Union for Conservation of Nature and Natural Resources since 2008 ( Abramov et al. 2016). Populations of M. sibirica are protected under the Appendix III of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (Convention for the International Trade of Endangered Species of Wild Fauna and Flora 2015) in India, the Tibet wildlife protection list, and the species is listed as “Near Threatened” under the China Red List ( Abramov et al. 2016). The species’ wide distribution leads conservationists to presume a large population with stable population sizes ( Abramov et al. 2016), but no demographic censuses have been undertaken.
Currently, there are no major threats to M. sibirica ( Abramov et al. 2016) . Historically, M. sibirica was considered a valuable furbearer in Siberia and China and used to make “kolinsky stable-hair” paintbrushes as well as ink brushes ( Heptner et al. 2001). However, hunting levels are currently low because of low commercial value of pelts ( Abramov et al. 2016). In addition, the placement of M. sibirica under Appendix III of CITES has placed restrictions on the importation of kolinsky brushes to some countries such as the United States ( Shaw 2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Mustela sibirica Pallas, 1773
| Law, Chris J. 2018 |
Kolonocus sibiricus peninsulae
| Kishida 1931: 380 |
Kolonocus sibiricus coreanus
| Domaniewski 1926: 55 |
Mustela hamptoni
| Thomas 1921: 500 |
hamptoni
| Thomas 1921: 500 |
Kolonocus sibirica sibirica
| Satunin 1914: 124 |
Kolonokus sibiricus australis
| Satunin 1911: 266 |
Lutreola major
| Hilzheimer 1910: 310 |
Lutreola tafeli
| Hilzheimer 1910: 310 |
major
| Hilzheimer 1910: 310 |
tafeli
| Hilzheimer 1910: 310 |
Lutreola quelpartis
| Thomas 1908: 53 |
quelpartis
| Thomas 1908: 53 |
Lutreola stegmanni
| Matschie 1907: 150 |
stegmanni
| Matschie 1907: 150 |
Putorius sibiricus noctis
| Barrett-Hamilton 1904: 391 |
Putorius davidianus
| Milne Edwards 1874: 343 |
Putorius moupinensis
| Milne Edwards 1874: 347 |
fontanierii
| Milne Edwards 1874: 205 |
moupinensis
| Milne Edwards 1874: 347 |
Putorius fontanierii
| Milne Edwards 1871: 205 |
Vison sibirica:
| Gray 1865: 117 |
Mustela hodgsoni
| Gray 1843: 118 |
Mustela horsfieldii
| Gray 1843: 118 |
hodgsoni
| Gray 1843: 118 |
horsfieldii
| Gray 1843: 118 |
Carnivora
| Bowdich 1821 |
Viverra sibirica:
| Shaw 1800: 431 |
Mustela sibirica
| : Pallas 1773: 701 |
Mustela sibirica
| : Pallas 1773 |
Mustela [Kolonocus] sibirica
| Pallas 1773 |
sibirica
| Pallas 1773: 701 |