Lynceus argillaphilus, Timms, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3702.6.1 |
|
publication LSID |
lsid:zoobank.org:pub:A875F2FF-3DAA-4AC3-9451-773F095A7C82 |
|
DOI |
https://doi.org/10.5281/zenodo.5463047 |
|
persistent identifier |
https://treatment.plazi.org/id/346D87FD-F132-2559-2A90-4DF5FEEC8186 |
|
treatment provided by |
Felipe |
|
scientific name |
Lynceus argillaphilus |
| status |
sp. nov. |
Lynceus argillaphilus View in CoL sp. nov.
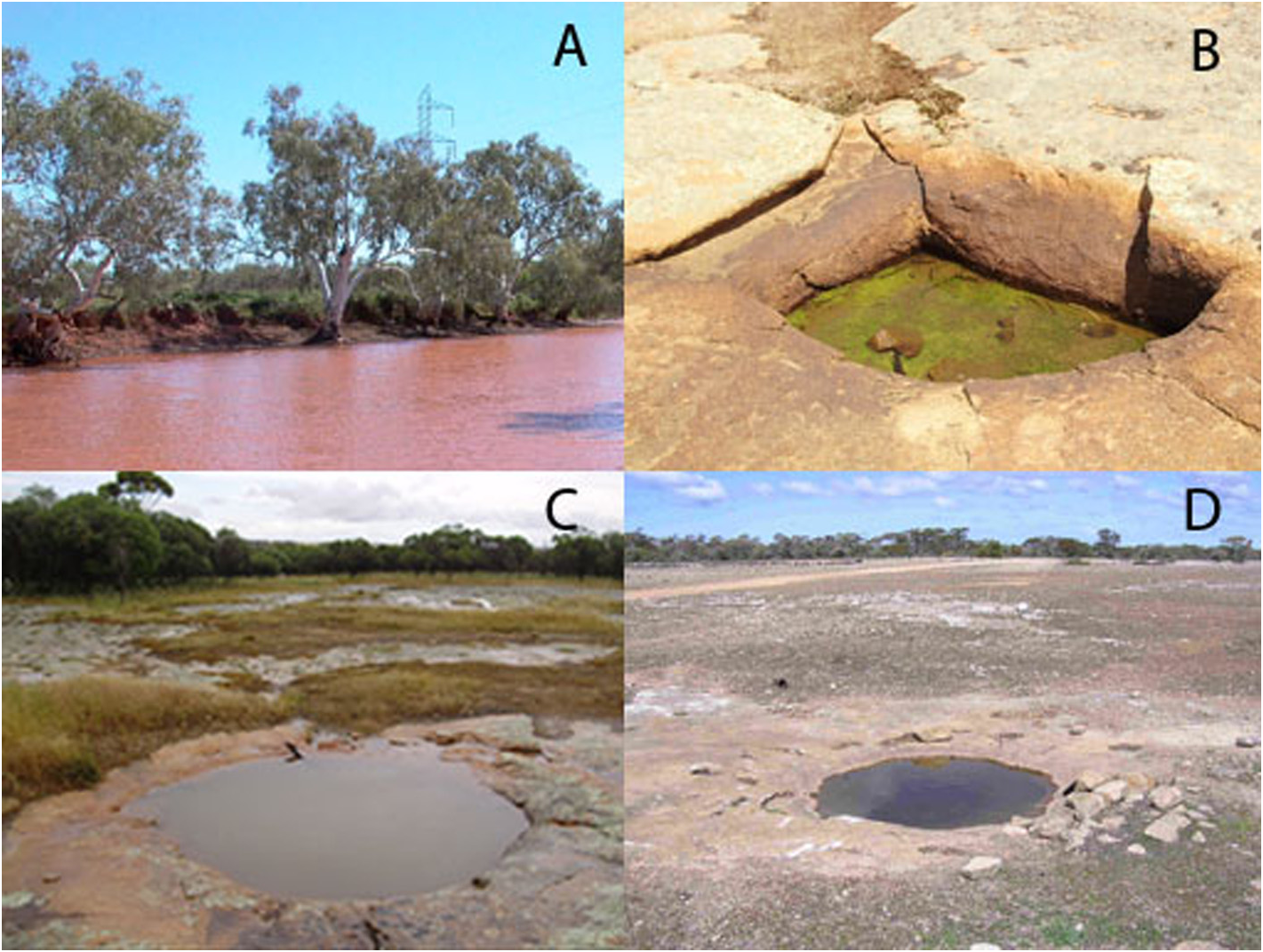
( Figs. 4C View FIGURE 4 ,7,8)
Etymology. The specific name is derived from the latin “ argilla ” meaning clayey and the greek “philein” meaning to love. Given the generic name Lynceus is male the specific epithet is “ argillaphilus ”. It refers to the finding of this species in highly turbid waters, an unusual habitat for Australian Lynceus , which usually live in clear waters.
Type Locality: Western Australia, Pilbara, 88.5 km E of Port Hedland, De Grey Claypan , 20 o 17’ 42”S, 119 o 25’ 21”E, 14 May 2004, A Pinder & H. Barron. This claypan is relatively large, turbid, intermittent and up to 1m deep. It is on cracking clay and surrounded by tussock grassland (Adian Pinder, pers. comm.) GoogleMaps
Hototype: Male deposited in Western Australian Museum, Perth, Length 5.5 mm, height 4.3 mm Registration number WAM C52151
Allotype. Female deposited in Western Australian Museum, Perth. Length 6.2 mm, height 5.2 mm Registration number WAM C52152
Other Material. Western Australia, Pilbara, 76 km SW of Port Hedland, pool in West Peawah Creek , 20 o 45’ 50”S, 118 o 00’ 47”E, 25 August 2005, A Pinder & J. McRae, stored in DEC’s collection GoogleMaps .
Diagnosis. Endite VI of male thoracopod 1 taller than endite III and with a very short digital process, reaching less than one quarter of the distance along the median margin of endite III. Endite V spathate and with lateral setae finely pectinate. Compound eyes of both sexes lying in a distinct depression and rostrum of both sexes strongly carinate. Female with lamina abdominalis with one anterior lobe, three dorsal lobes and two to three lateral lobes.
Description. Male: Head ( Fig 7B,C View FIGURE 7 ) a little smaller than body. Fornices angulate and weakly arcuate over second antennal base. Posterior fornices a little narrower than widest part of head near the eyes. Small mound centroposteriorly, the site of the dorsal organ. Compound eyes close together in a depression about halfway along central ridge and just posterior to the frontal pore and two lateral setal fields. Suture and ridge posterior to these fields strongly developed, and small transverse ridge anterior to the setal fields. Ocellus deeply embedded beneath the setal fields. Rostrum about twice as long as wide, but varying in width with narrowest part just anterior to the setal fields and widest terminally. Rostrum strongly carinate and truncated at right angles to linear axis. Rostrum truncated at almost 90 o to linear axis and with whole truncated surface strongly cilated. Head bent anteriorly from linear axis by about 45 o mainly at rostral base.
First antenna ( Fig. 7D View FIGURE 7 ) slightly longer than rostrum and with two subequal cylindrical antennomeres. Distal antennomere bears olfactory setae, sparsely dorsally and many apically.
Second antenna ( Fig. 7E View FIGURE 7 ) biramous, well developed and protruding about a third of its length beyond the rostrum. Peduncle of three segments, proximal segment with three to four setae and both middle and distal segment without spines. Anterior (dorsal) ramus with about 22 antennomeres and ventral ramus about 28 antennomeres. Both rami with plumose ventral setae, one per antennomere and dorsal ramus with dorsal setae as well.
Labrum large, well developed, clothed in small setae. Mandible broadly spatulate. First maxilla typical for genus and second maxilla absent.
Carapace ( Fig 7A View FIGURE 7 ). Umbo absent and abductor muscle scar in an anteriolateral position about 1.5 times its diameter from the margin and associated with oval imprint of maxillary glands l at about 30 o to the hinge line. Hinge line weakly convex, anterior valve slightly more evenly rounded than posterior valve, eye area of head often slightly protruding between valves. Valves rounded and inflated laterally.
Thorax. Ten thoracic segments, each with a pair of thoracopods. Anal plate partly divided medially, each with apex rounded and bearing a long seta. Somite below divided and with subacute apex.
Thoracopod I ( Fig. 7F View FIGURE 7 , 8A–C View FIGURE 8 ) modified as a clasping appendage, right and left claspers equal in size and shape. Endite VI compressed making it subequal in length to endite III and longer than it is wide; its digitiform process short, compact and protruding less than one quarter along medial margin of endite III. Endite V spathate, the distal third up to three times the width of the basal third. Endite V with a row of about 12 lateral setae, each finely pectinate, and a field of lithe setae almost opposite the lateral row. Endite IV almost symmetrical, small at about one tenth size of endite V and clothed in long, lithe setae. Endite III rectangular but with median margin arcuate. Distomedial corner with a row of about six rectangular spines of uniform size with arcuate distal margins. Mediolateral face with numerous stout setae, longest and most crowded in distomedial corner.
Thoracopod II similar to that of L. baylyi n. sp. Only two to three weakly pectinate setae on apices of of endites IV, V and the palp ( Fig. 7G View FIGURE 7 ) though many short, stouter anterior setae on endite II.
Subsequent thoracopods similar to thoracopod II, though the last three much reduced in size and lacking epipodites and proximal lobe of the exopodites.
Female. Head ( Fig. 7J View FIGURE 7 ): general structure similar to male.. Strong anterior dorsal carina, rostrum about twice as long than wide and anterior margin evenly arcuate. Head bent anteriorly from linear axis by about 45 o mainly at rostral base. Both antennae of similar structure to male, but first antenna not reaching rostral apex, and second antenna less protruding as rostrum is not truncated in female.
Carapace as in male, umbo lacking, same shape and size. Egg mass, if present visible through the carapace.
Thorax. Twelve thoracic segments, the last three with a lamina abdominalis ( Fig. 7K View FIGURE 7 ) with a long digital process anteriorly, three shorter and curved digital processes medially, two more processes of similar structure laterally and another somewhat foliaceous process ventrolaterally.
Thoracopods: Twelve pairs of thoracopods, IX and X with exopod dorsal lobes cylindrical and extending dorsally beyond thoracic dorsum. These help to anchor the egg mass. Last five thoracopods much reduced without an epipodite and proximal exopodite.
Resting egg: ( Fig. 4C View FIGURE 4 ) Round, irregular low ridges enclosing vaguely circular depressions. Size 103.4 ± 3.8 ųm (n = 5)
Characteristics of the habitat. Water is moderately turbid (Nephelometric Turbidity Units 84) and the other site at Peewah Ck ( Fig. 9A View FIGURE 9 ) extremely turbid (NTU 1100) (Adrian Pinder, pers.comm.), both being unusual habitats for Lynceus in Australia.
Distribution. This species probably does not occur in other areas of Western Australia and is uncommon as these are the only two sites known in the Western Australia, much of which has been studied intensively for aquatic invertebrates in recent years ( Halse et al., 1998, 2000; Pinder et al., 2004, 2010, 1012).
Comments. Little is known of this species in that only four specimens from two sites in coastal Pilbara were available for study. The male clasper and female lamina adominalis are particularly distinctive, while there are many minor differences from most other Australian species, including the sunken eyes, fully ciliated truncated surface of the male rostrum, the sharply carinate rostrum, the lack of spines on the third peduncle antennomere, and the low number of pectinate spines on endites III–V of thoracopod II. In that these later spines are used for scraping food off hard surfaces ( Fryer and Boxshall, 2009), their reduction could be an adaptation for living in an environment of soft clayey surfaces, rather than in the usual rocky gnammas or vegetated pools. There has been no reduction in eye size, as in the anostracan Branchinella pinderi also living in extremely turbid pools in the Pilbara (Timms, 2008).
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |