Chasmodia Macleay, 1819
|
publication ID |
https://doi.org/ 10.1649/0010-065X(2001)055[0385:DOTLOC]2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/340B87F4-FF63-C624-FDD6-FF0DFCB4F986 |
|
treatment provided by |
Tatiana |
|
scientific name |
Chasmodia Macleay |
| status |
|
Larva of Chasmodia Macleay
Based on C. collaris , the larvae of Chasmodia are most similar to those of Macraspis and Chlorota . Larvae of Chasmodia and Macraspis are separated based on the metathoracic claws (in Chasmodia the metatarsal claws have a shortened apical process, in Macraspis the metatarsungulus is greatly reduced and poorly sclerotized in comparison to the tarsunguli of the pro and mesothoracic legs), and the width of the septula (clearly defined on the lower anal lip of Macraspis larva, vaguely defined in the same area of Chasmodia larva). Larvae of Chlorota are separated from larvae of Chasmodia based on the ocellus (lacking in Chlorota ); number of dorsal sensory spots of the last antennal segment (six in Chasmodia , four in Chlorota ); number of maxillary stridulatory teeth (five in Chasmodia , seven in Chlorota ); setae of the disc of the labium that are moderately long and slender (short and stout setae in Chlorota ), and the disc of the lateral lobe of the labium which has a random field of setae in Chasmodia (with a line of setae on the discal region in Chlorota ).
The genus Chasmodia is exclusively Neotropical and includes approximately 31 species ( Machatschke 1972; Delgado 1997) distributed from southern Mexico to Paraguay, middle Argentina, and southern Brazil. Two species of Chasmodia occur in Mexico: C. collaris Blanchard and C. jamesonae Delgado. Chasmodia collaris is distributed from southern Mexico to Panama where it inhabits tropical perennial forests and subperennial forests at elevations between 100–1,400 m ( Morón et al. 1997). Adults have been observed feeding on Piper spp. (Piperaceae) and have been found in the soil in May and September ( Morón et al. 1997).
The description of C. collaris larva is based on one cast skin that is broken and partially distorted. Thus, some characters were not easily observable.
ThirdInstar Larva of Chasmodia collaris Blanchard Figs. 19–31 View Figs
Specimens studied. One cast skin of a thirdinstar larva and associated adult with the following data: MEXICO: Chiapas , Montes Azules, Naja´, 18I1979, unidentified rotten log, 700 m, J. Valenzuela. The cast skin was obtained by rearing the thirdinstar larva in rotting wood. The pupal stage lasted 35 days at 24°C, and the adult was collected immediately. Specimens housed at the Instituto de Ecología , Xalapa, Mèxico .
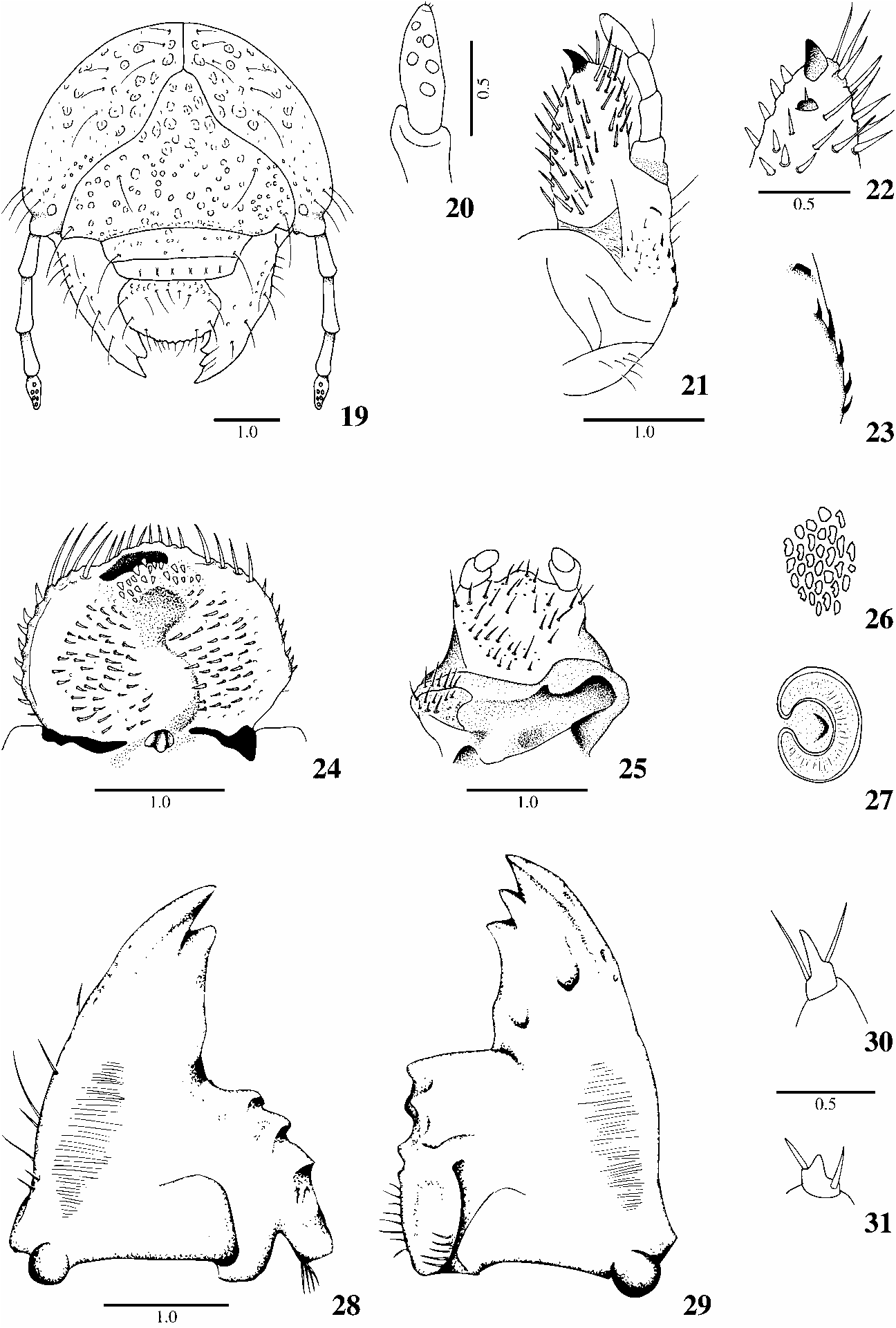
Description. Head. Cranium ( Fig. 19 View Figs ): Maximum width of head capsule 4.7 mm. Surface of cranium unicolorous, yellowishorange. Surface of cranium deeply punctate near clypeus, shallowly punctatereticulate near epicranium, shallow and randomly punctate on base of clypeus, and rugopunctate on borders of labrum and anterior face of mandibles. Frons ( Fig. 19 View Figs ) without anterior frontal setae and exterior frontal setae and with 1 long, posterior frontal seta on each side; each anterior angle of frons with 1 long seta; remaining cranial surface with 3 dorsoepicranial setae, 3 epicranial setae and 3 paraocellar setae on each side. Ocelli small, poorly defined. Anterior tentorial pits not apparent. Clypeus: 1 long, lateral seta present on each side. Labrum: Form slightly asymmetrical, left lateral margin widely angulate; 5 setae near base; without central setae; 3 lateral setae at each side. Epipharynx ( Fig. 24 View Figs ): Haptomerum entire, prominent, with 2 irregular rows of short, conical heli; each row with 10 heli. Zygum wide, not extended at sides. Epizygum absent. Chaetoparia with sparse sensilla; right chaetoparia with 54 spinelike setae; left chaetoparia with 51 spinelike setae; left acanthoparia with 9 short, spinelike setae; right acanthoparia with 8 short, spinelike setae; acroparia with 8 thick, long setae; pedium clear, wide; gymnoparia narrow, without plegmatia; dexiotorma sinuose; sclerotized plate poorly sclerotized; sensory cone surrounded by strongly scler otized, wide plate; haptolachus with scattered sensilla; laeotorma sinuous, narrowed toward epitorma, with rounded apex; pternotorma wide, rounded, completely fused with laeotorma; dexiophoba and laeophoba absent. Right Mandible ( Fig. 28 View Figs ): Scissorial area shortened, with 2 strong acute, apical teeth; molar area with 3 wide convex, ridged lobes; calx truncated; brustia formed by 4 setae; ventral process wide, rounded. Left Mandible ( Fig. 29 View Figs ): Scissorial area shortened, with 3 shortened acute, apical teeth; molar area with prominent, sharp, distal lobe and ridged basal lobe; acia absent; brustia absent; ventral process slightly narrowed, rounded. Ventral stridulatory area of both mandibles elongat ed, narrowed toward their ends, formed by microscopic, transverse ridges; without setose punctures near stridulatory area. Maxilla ( Figs. 21–22 View Figs ): Galea ( Figs. 21–22 View Figs ) with 1 welldeveloped terminal uncus. Lacinia with 1 reduced uncus and a minute seta. Maxillary terminal unci surrounded by 4 long, dorsolateral, stout, sclerotized setae, 3 ventral heli, and 2 mesal heli. Maxillary stridulatory area with regular row of 5 long, sharply pointed teeth and with anterior process ( Fig. 23 View Figs ). Labium ( Fig. 25 View Figs ): Hypopharyngeal sclerome asymmetrical, strongly produced on right side into toothlike, curved process. Anterior border of labial glossa medially prominent. Antennae ( Fig. 20 View Figs ): Segments moderately enlarged; ventral process at distal border of 3rd segment weakly developed, with rounded apex. Dorsal surface of last antennal segment with 6 oval or rounded sensory spots ( Fig. 3b). Ventral surface of last antennal segment with 7 oval sensory spots. Thorax. Thoracic spiracles 0.5 mm long and 0.6 mm wide; respiratory plate reddish brown, regularly shaped as a closed ‘‘C’’, with 16–22 irregularly shaped holes ( Fig. 26 View Figs ) across diameter. Spiracular bulla slightly prominent, rounded. Distance between lobes of respiratory plate shorter than dorsoventral diameter of the bulla. Pronotum with distinct, yellowish, broad, irregularly shaped, lateral scleromes, without setae at each side. Dorsum of prothorax with transverse row of 16 long, slender setae; mesoprescutum with transverse, irregular row of 12 long, slender setae; mesoscutellum with transverse row of 12 long, slender setae; metaprescutum with 6 long, slender setae; metascutellum with 16 long, slender setae. Legs ( Figs. 30–31 View Figs ): Tarsal claws on pro and mesothoracic legs with sharply pointed apical process, 1 external preapical seta and 1 internal basal seta ( Fig. 30 View Figs ). Tarsal claws on metathoracic legs reduced, with subconical apical process, and with 2 long setae ( Fig. 31 View Figs ). Abdomen. Spiracles on abdominal segment I 0.39 mm long and 0.52 mm wide; spiracles on segments II–III slightly wider than preceding, 0.42–0.43 mm long and 0.60–0.61 mm wide; spiracles on segments IV–VII slightly narrower than preceding, 0.41–0.50 mm long and 0.51–0.53 mm wide; spiracles on abdominal segment VIII small er than preceding, 0.42 mm long and 0.43 mm wide. Respiratory plates reddish brown, regularly shaped as a closed ‘‘C’’; spiracular bulla slightly prominent, rounded; distance between lobes of respiratory plate shorter than dorsoventral diameter of bulla. Dorsa of segments I–VI each with 1 irregular, transverse row of 20–24 long, slender setae and many short, spinelike setae; dorsum of segment VII with transverse row of 20 long, slender setae and sparse, spinelike setae, dorsa of segments VIII–IX with 2 irregular, transverse rows of 12–20 long, slender setae; dorsum of segment X with 2 vaguely defined, transverse rows of 6–10 long, slender setae and 28 scattered, long, slender setae. Raster with 25–28 short and moderately long, mixed setae on campus; tegilla formed by 20–24 long and short mixed setae on each side; barbula formed by 4–5 long setae on each side; lower anal lip with 40–50 long, slender setae at each side of border; with poorly defined septula and irregular palidia formed by 13–16 pali extended on lower anal lip. Anal lip weakly curved. Approximate dorsal body length not measurable.
Remarks. The larva of C. collaris is the first larva described in the genus Chasmodia . The thirdinstar larva was collected from rotting logs on 18 January, pupated on 21 February, and the adult emerged on 28 March, 1978.
Acknowledgments We thank Pedro ReyesCastillo and Jorge Valenzuela (Instituto de EcologÌa, Xalapa, México) for donation of the larval specimens studied in this paper. We thank Angie Fox (Scientific Illustrator, University of Nebraska) for her assistance with the illustrations and Brett C. Ratcliffe (University of Nebraska) for critically reviewing the manuscript. Thanks also to two anonymous review
ers for their valuable comments. This project was supported by an NSF/PEET grant (DEB9712447) to B. C. Ratcliffe and M. L. Jameson.
Literature Cited
Costa, C., S. A. Vanin, and S. A. CasariChen. 1988. Larvas de Coleoptera do Brasil. Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil.
Delgado, L. 1997. Una especie nueva de Chasmodia del tropico del Pacifico Mexicano (Coleoptera: Melolonthidae; Rutelinae; Rutelini). Folia Entomologica Mexicana 100: 15–21.
Jameson, M. L., B. C. Ratcliffe, and M. A. Morón. 1994. A synopsis of the Neotropical genus Calomacraspis Bates with a key to larvae of the American genera of Rutelini (Coleoptera: Scarabaeidae: Rutelini). Annals of the Entomological Society of America 87: 43–58.
Jameson, M. L. 1996. Revision and phylogeny of the Neotropical genus Cnemida (Coleoptera: Scarabaeidae: Rutelinae). Insecta Mundi 10: 285–315.
Jameson, M. L. 1999 (1998). Phylogenetic analysis of the subtribe Rutelina and revisions of the Rutela generic groups (Coleoptera: Scarabaeidae: Rutelini). Bulletin of the University of Nebraska State Museum 14: 1–184.
Machatschke, J. W. 1972. Scarabaeoidea: Melolonthidae, Rutelinae. Coleopterorum Catalogus Supplementa 66 (1): 1–361.
Monne´, M. A. 1969. Descripción del último estadio larval de ‘‘ Macraspis dichroa cribrata ’’ Waterh., ’’ Blaesia atra ’’ Burm. y ’’ Marmarina tigrina ’’ (Gory and Perch.) (Coleoptera, Scarabaeidae). Revista Brasiliera Biologia 29: 367–376.
Morón, M. A. 1976 a. Descripción de las larvas de tres especies mexicanas de Pelidnotinos (Coleoptera; Melolonthidae, Rutelinae) y algunas observaciones sobre su biología. Anales del Instituto de Biologia, Universidad Nacional Autonoma de Mexico, Serie Zoologia 47: 7–18.
Morón, M. A. 1976 b. Descripción de las larvas de tres especies mexicanas de Melolonthinos (Coleoptera; Melolonthidae, Dynastinae y Rutelinae). Anales Instituto Biologia Universidad Nacional Autonomia Méxicana Series Zoologia 47: 119– 134.
Morón, M. A. 1983. A revision of the subtribe Heterosternina (Coleoptera: Melolothidae, Rutelinae). Folia Entomologica Mexicana 55: 31–101.
Morón, M. A. 1993. Observaciones comparativas sobre la morfología pupal de los Coleoptera Melolonthidae neotropicales. Giornale Italiano Entomologia 6: 249– 255.
Morón, M. A., and C. Deloya. 1991. Los Coleópteros Lamelicornios de la Reserva de la Biosfera ‘‘La Michilía,’’ Durango, México. Folia Entomologica Mexicana 81: 209–283.
Morón, Miguel A., and Guillermo Noguiera. In press. Third stage larva and pupa of Paraheterosternus luedecki (Becker) (Coleoptera: Melolonthidae; Rutelinae). Journal of the Kansas Entomological Society.
Morón, M. A., B. C. Ratcliffe, and C. Deloya. 1997. Atlas de los Escarabajos de México. Coleoptera: Lamellicornia, Vol. 1 Familia Melolonthidae, subfamilias Rutelinae, Dynastinae, Cetoniinae, Trichiinae, Valginae y Melolonthinae. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), Sociedad Mexicana de Entomología, A.C., Xalapa, Mexico.
Ohaus, F. 1934. Coleoptera Lamellicornia. Fam. Scarabaeidae, Subfam. Rutelinae. Genera Insectorum, Fasc. 199A: 1–172.
Ritcher, P. O. 1966. White Grubs and their allies: a study of North American Scarabaeoid larvae. Oregon State University Press, Corvallis.
Solís, A., and M. A. Morón. 1998. Neotropical genus Platyrutela Bates (Coleoptera: Melolonthindae). Annals Entomological Society of America 3: 269–278.
Vanin, S. A., and C. Costa. 1980. Larvae of Neotropical Coleoptera. III. Scarabaeidae, Rutelinae. Papéis Avulsos de Zoologia (Sao Paulo) 33: 275–282.
(Received 16 April 2000; accepted 15 August 2000)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.