Squalius janae, Bogutskaya & Zupančič, 2010
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2536.1.3 |
|
persistent identifier |
https://treatment.plazi.org/id/2E0E878F-6632-442A-FE87-56A2FE90F88E |
|
treatment provided by |
Felipe |
|
scientific name |
Squalius janae |
| status |
sp. nov. |
Squalius janae View in CoL , sp. nov.
( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 , 4a View FIGURE 4 , 8a View FIGURE 8 )
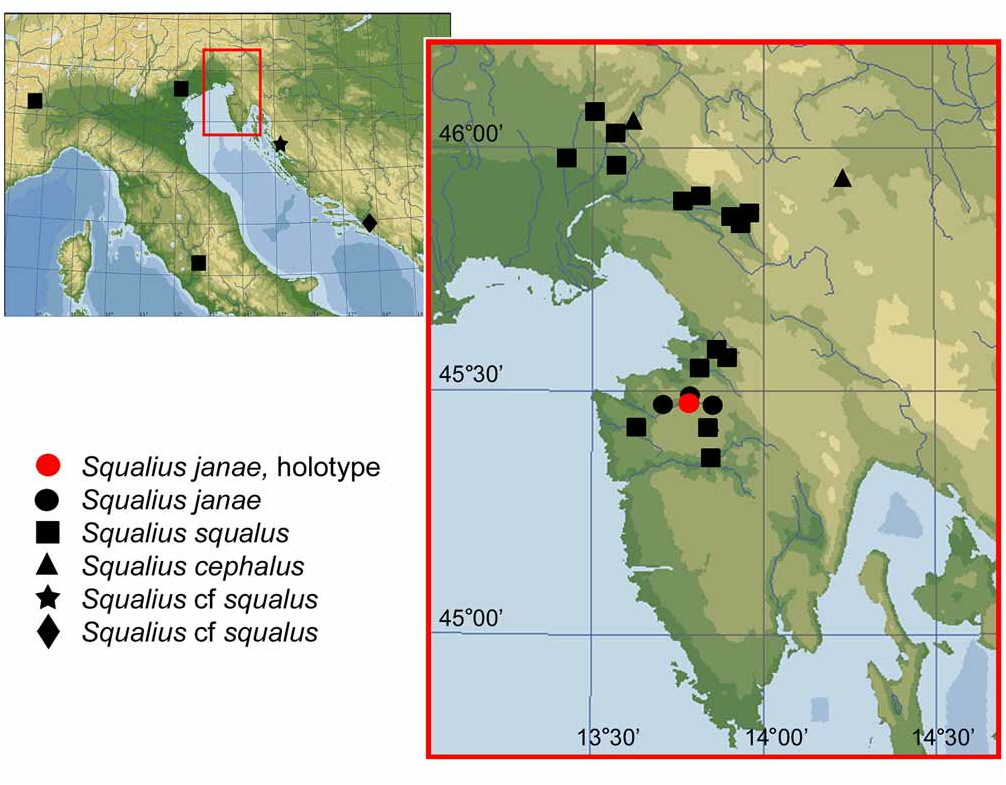
Holotype ( Fig. 1 View FIGURE 1 ): SMNH 207, 183.7 mm SL; SLOVENIA: Dragonja River about 2 km upstream from bridge on road from Župančiči, 45°28’N 13°46’E; coll. Zupančič, 1 Aug. 2008. GoogleMaps
Paratypes. PZC 475 , 6 , 85.9–174.9 mm SL; same data as holotype GoogleMaps . PZC 452 , 6 , 74.9–132.5 mm SL; SLOVENIA: Dragonja River at bridge on road from Župančiči, 45°28’15”N 13°45’11”E; coll. Zupančič, 24 Nov. 2007 GoogleMaps . PZC 453 , 6 , 66.2–157.6 mm SL; same locality; coll. Zupančič, 17 May 2009 GoogleMaps . PZC 454 , 19 , 85.8 – 158.0 mm SL; SLOVENIA: Dragonja River downstream from confluence with Pinjevec [Rokava] River, 45°28’30”N 13°44’31”E; coll. Zupančič, 10 Apr. 2009 GoogleMaps . PZC 455 , 7 , 68.4–212.7 mm SL; SLOVENIA: Dragonja River south from Koštabona, 45°28’20”N 13°44’11”E; coll. Zupančič & Naseka, 5 July 2008 GoogleMaps . PZC 456 , 8 , 111.2 –190.0 mm SL; SLOVENIA: Dragonja River drainage: Pinjevec River at Župančiči, 45°29’N 13°46’E; coll. Zupančič, 1 Feb. 2007 GoogleMaps . PZC 476 , 4 , 80.4 –120.0 mm SL; same locality and collector; 24 Nov. 2007 GoogleMaps . ZISP 54690 View Materials , 11 View Materials , 88.5–149.3 mm SL; SLOVENIA: Dragonja River south from Koštabona, 45°28’20”N 13°44’11”E; coll. Zupančič & Naseka, 5 July 2008 GoogleMaps .
Diagnosis. Squalius janae is distinguished from other species of the genus Squalius in the Adriatic basin by a combination of characters: a long head, head length, 29−32% SL, always markedly greater than body depth; a pointed conical snout; a slightly subterminal mouth with a clearly projecting upper jaw; a straight, oblique mouth cleft; the lower-jaw length, 39−46% HL, exceeding the caudal-peduncle depth; a large eye, its diameter 19−25% HL; a large triangular 5 th infraorbital; a marked discontinuity between the dorsal profile of head and body; usually 44−47 total latera-line scales; usually 9½ branched anal-fin rays; usually 44 total vertebrae, vertebral formulae 24+20 and 25+19; a strong silvery tint in body colouration; scales easily lost; iris, pectoral, pelvic and anal-fin pigmentation with yellow shades; flank scales margined by a few black pigment dots along their free margin and intense black pigments on the scale pockets, forming black, vertically elongate spots.
Description. See Table for morphometric data of holotype and 42 paratypes. The body is elongate, moderately compressed. The body is not deep: depth at the dorsal-fin origin is 20−26% SL. There is a marked discontinuity between the dorsal profile of the head and the body, and the dorsal profile behind the hump is often straight.
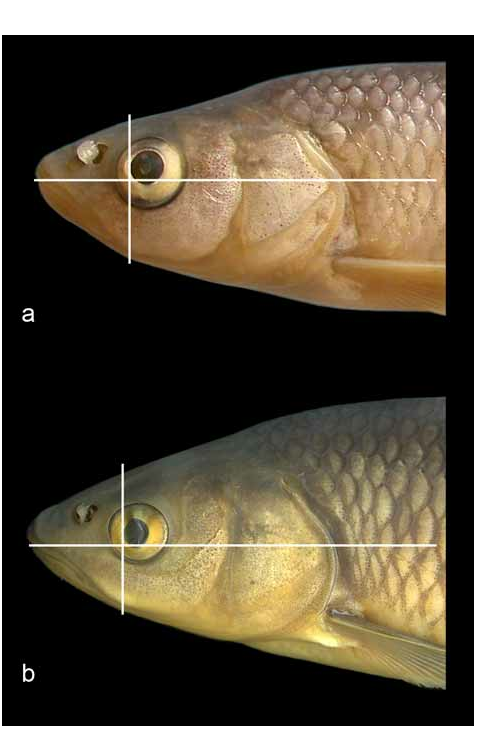
The head is long, its length, 29−32% SL, is always greater than the body depth (111−137% of the latter), and is 2.6–3.0 times the caudal-peduncle depth. The head depth at the nape is 59–64% HL. The upper head profile is almost straight, and the snout is pointed. The mouth is subterminal or almost terminal, though never clearly terminal for the uppermost point of the mouth cleft is always below the level of the middle of the eye: from the lower margin of the pupil to the lower margin of the eye ( Fig. 4a View FIGURE 4 ). The lower jaw-quadrate junction lies on the vertical through the anterior margin of the pupil or behind it, often on the vertical through the middle of the eye. There is no marked chin formed by the mandibular symphysis. The upper jaw clearly projects beyond the lower jaw, often includes the tip of the lower jaw. The mouth cleft is straight, oblique, and the lower jaw-quadrate junction is usually clearly visible, forming a distinctly obtuse angle. The mouth is large and both jaws are long. The upper-jaw length, 31–37% HL, is only slightly shorter or about equal to the caudal-peduncle depth. The lower-jaw length, 39–45% HL, exceeds the caudal-peduncle depth by 1.1–1.3 times. The width of the lower jaw is always greater than both the operculum depth and the interorbital width. The interorbital space is moderately wide, its width averaging 37% HL, and the operculum is not deep, 33– 39% HL, that corresponds to a relatively small head depth at nape, 59–64% HL. The eye is large, its horizontal diameter being 19−25% HL, and 51−70% the interorbital width. The smallest eye diameter, 19% HL, is found in the largest specimen 190.6 mm SL.
The dorsal fin has 3 simple and 8½ branched rays in all specimens. Its outer margin is straight. The dorsal fin is located slightly behind the end of the pelvic-fin base. The dorso-hypural distance falls on about the middle of the eye, rarely behind the posterior eye margin, or slightly in front of the eye. The dorsal fin is high, its depth 16–20% SL. The anal fin has 3 simple and 9½ branched rays; 8½ branched rays were found only in three paratypes. The anal-fin outer margin is slightly to markedly convex. The caudal fin is deeply forked, its lobes slightly pointed.
The number of gill rakers (in total on the outer side of the first left gill arch) is 9 in the holotype; 8 (24), 9 (20) or 10 (4) in the examined paratypes. The 10 examined paratypes had 2.5–5.2 hooked, serrated pharyngeal teeth.
The lateral line is complete with none to 3, usually 1, unpored scales at the posterior end of the lateral series. The total number of lateral line scales is 46 in the holotype; 40 (1), 42 (1), 43 (3), 44 (15), 45 (20), 46 (16) or 47 (4) in the paratypes. A pelvic axillary scale is present. The scales on the flanks are easily lost.
The general topography of cephalic sensory canals is typical of Leuciscus s.l., as described by Bogutskaya (1988). The CSO has 11–14 pores; the posterior section of the canal is elongated, downwardly bent, passing close to the CST, and usually having two canal segments on the parietal. There are 4–6 canal openings on the nasal and (6) 7–9 on the frontal. The CIO has (16, 17) 18–23 pores with (5) 6–8 canal openings on the 1st infraorbital. The CPM has 15–20 pores. There are (5) 6–8 canal openings on the dentary. The CST has 7–11 pores.
The total vertebrae including four Weberian vertebrae and the last complex centrum are 44 in the holotype, 43 (3) or 44 (27) in the paratypes examined; the mean is 43.9. The number of abdominal vertebrae (including intermediate ones; precaudal vertebrae auctorum) is 25 in the holotype, 24 (10) or 25 (9) in the paratypes examined; the mean is 24.5. The number of predorsal vertebrae (anterior to the first dorsal pterygiophore) is 15 in the holotype, 15 (18) or 16 (1) in the paratypes examined; the mean is 15.05. Intermediate vertebrae are 5 in all specimens. The number of caudal vertebrae is 19 in the holotype, 18 (3), 19 (7) or 20 (10) in the paratypes examined; the mean is 19.4. The vertebral formulae are 25+19 (in holotype and 7 paratypes), 24+20 (in 10 paratypes) or 25+18 (in 3 paratypes).
The cranium is very similar to that described for ‘ L. cephalus ’ in Bogutskaya and Zupančič (1999). The neurocranium is broad, markedly depressed especially in the orbital region: depth of the ethmoid region is 11– 12%, the depth of the occipital region is 31–32%, the width of the neurocranium between the lateral margins of the lateral ethmoids is 40–42%, the width of the neurocranium between the lateral margins of the sphenotic lateral processes is 49–50%, and width of the neurocranium between the lateral margins of the pterotics (maximum cranial width) is 56–58% neurocranial base length. The interorbital septum is almost or completely reduced. In undissected specimens, maximum cranial width is 71–79% cranial roof length (mean 75.73, SD 2.69), and the width of the supraethmoid is 27–34% cranial roof length (mean 30.95, SD 2.26). The vomer is markedly shortened, much wider than long. The preethmoid is completely cartilaginous. The paired pterosphenoids contact along the anterior margin of the orbital-hypophyseal foramen. The sphenotic contributes to the apex of the inner wall of the subtemporal fossa. The carotid foramen is very small. The 4 th and 5 th infraorbitals are extensive, the 5 th one is roughly triangular and covers the most part of or the entire outer surface of the dilatator fossa. The bones of jaws are markedly elongated. The maxillary ascending dorsal process is shallow and broad. The length of the dentary is 46–49% neurocranial base length.
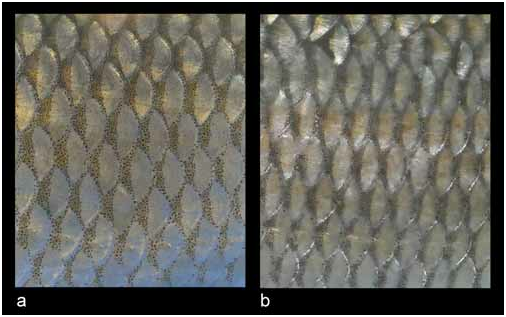
Colouration. In live specimens, the overall colouration has a strong silvery tint, and the back is only slightly darker than the flanks and the belly ( Fig. 3 View FIGURE 3 ). The back and flank scales are margined by few black pigment dots along their free margin with most pigments concentrating on scale pockets ( Fig. 8a View FIGURE 8 ). No conspicuous dark reticulated pattern is seen in live specimens. The iris, pectoral, pelvic and anal-fin pigmentation is with yellow shades. Juveniles have a very light colouration and hyaline fins while adults in April–July (specimens over about 100 mm SL) have the colouration most intense—the yellow colour in the iris and the anal fin is most distinctive, the anal fin in its lower part has darker pigmentation sometimes forming a roundish spot; black pigment in general is located on the fin membrane along the rays with the central part of the membrane pale. Formalin fixed and ethanol preserved specimens retain silvery grey colouration with no brownish or bronze shades; yellow pigments are often lost. The pigmentation on scales is much more distinct than in live specimens although the main pattern is retained, characterised by weak pigmentation along scale outer margins while that on scale pockets form black, vertically elongate spots.
Distribution. Squalius janae is definitively known only from the River Dragonja drainage of Slovenia and Croatia.
Habitat and biology. The specimens of the new species were collected from small rivers which become shallow in summer, often partly dried, where fish survive in remaining pools. The river flows over a bare limestone bed ( Fig. 9 View FIGURE 9 ) alternating with silted pools. The Dragonja section examined on 6 August 2009 had clear and colourless water, a temperature of 21.4°C, pH 8.8, a mineralization of 0.32 gl -1, and a slow current. No other fishes were caught in the stream together with S. janae . Adults with completely ripe gonads, both females and males, were found in the middle of May and at the beginning of July; post-spawning adults were among the specimens examined in July. The smallest mature male was 101.4 mm SL. Adult males retain the nuptial tubercles until at least August. With regard to digestive-tract content, specimens from the sample collected on April 10, 2009, upstream from the confluence of Dragonja and Pinjevec, were slender and contained no food, while specimens collected on the same date but 5 km downstream were fat and rotund, and contained algae, plant remains and detritus. This may reflect a difference coincident with the spring water temperature rise and start of plant growth.
Etymology. The species is named after Jana Zupančič whose enormous patience and assistance made this study possible.
Comparative remarks. The new species belongs to the Squalius cephalus group defined on morphological characters, which is characterized by a higher number of cephalic canal pores and expanded 4 th and 5 th infraorbital. Thus, Squalius janae differs from S. aradensis and S. torgalensis (both from southern Portugal) by possessing more numerous vertebrae—43−44 total, 24−25 abdominal and 18−20 caudal (vs. 36− 38, 20−21 and 16−17, respectively). It can be further distinguished from these two species and S. malacitanus (Malaga area in Spain; data for this species is taken from Doadrio and Carmona (2006)) in having more numerous total lateral line scales, usually 43−48 (vs. 35–43) and 9½ anal-fin branched rays (vs. 7−8½). Squalius janae is different from S. valentinus (south-eastern Spain; data for this species is taken from Doadrio and Carmona (2006)) by having 9½ anal-fin branched rays (vs. usually 8½) and usually 43−48 total lateralline scales (vs. 35−39), from S. carolitertii (north-western Iberian Peninsula) by a terminal or only slightly subterminal mouth (vs. clearly subterminal), a pointed projected snout (vs. rounded) and the lower jaw length exceeding the body depth (vs. less than body depth), more numerous vertebrae, usually 25+19 or 24+20 (vs. usually 23+18), and expanded 4 th and 5 th infraorbitals (vs. narrow), and from S. castellanus (Tagus River in Spain; data from Doadrio et al. (2007b)) by usually 43−48 total lateral-line scales (vs. 39−42), and a long head, 27−32% SL (vs. short, 20−23% SL).
Among the S. cephalus species group, the new species shares with S. pyrenaicus (southern and central Iberian Peninsula) such characters as a terminal mouth with a pointed snout, a dark pigment mark on scale pocket of each flank scale, expanded infraorbitals, and a wide supraethmoid, but differs in having more numerous vertebrae—43−44 total, 24−25 abdominal and 18−20 caudal (vs. 37−40, 21−23 and 16−18). From S. laietanus (Ebro to Port Bou drainages in Spain, and the Tech and Agly drainages in France; data from Doadrio et al. (2007a)), the new species differs by the absence of brown pigments on the inner area of the scales and the absence of any longitudinal stripes (vs. presence), a marked discontinuity between the dorsal profile of the head and the trunk (vs. no or only a weak discontinuity), the dorso-hypural distance falling in front of the posterior margin of the eye when reported forwards (vs. at or behind), and 9½ branched anal-fin rays (vs. 8½).
The new species differs from S. cephalus (most of Europe north from the Pyrenees, the Alps, the Dinarides, and the Caucasus) by having usually 9½ anal-fin branched rays (vs. 8½), yellow pigmentation in the iris, pelvic and anal fins (vs. intense red or orange), the presence of black pigmentation of the anal-fin in adults (vs. the absence), a weak row of black pigments along the free margin of flank scales and a dense pigment on scale pockets that do not form a regular reticulate pattern (vs. a well-pronounced row along the margin forming a regular reticulate pattern). As it can be seen from the list of comparative material, S. cephalus is also found in the Soča drainage (Rohat at Deskle), but this most probably represents an introduction.
A key issue for designating Squalius janae as a new species is a comparison with S. squalus . The new species can be distinguished from S. squalus from the Apennine Peninsula by a set of characters which include the following ones discussed in greater detail. Morphometric data can be compared from the Table. In general, S. janae has a shallower body, the body depth at the dorsal-fin origin being 20−26% SL (mean 23.2), while in S. squalus from Po and Soča the body is deeper, 23−28% SL (means 26.1 and 25.4 in the Po− Soča and Osapska samples, respectively, and 25.8 for the combined sample); the difference is statistically significant. The ranges of the dorsal-fin depth overlap, too, but the difference is statistically significant. The head is markedly longer in S. janae than in S. squalus : head length is 29−32% SL (mean 29.4) in contrast to 25−29% SL in S. squalus , and equals to 111−137% body depth (mean 123.1) while in the latter species it is 94−120.5% body depth (means 105.8 and 109.3, and 107.2 for the combined sample). The depth of the caudal peduncle falls 2.6−3.0 times in the head length in S. janae while in S. squalus the ratio is 2.2−2.8. An easy useful character to distinguish the two species is relative length of the lower jaw—it rarely equals but usually clearly exceeds the caudal-peduncle depth in S. janae while it is about equal to or less than the caudal peduncle depth in S. squalus . The lower-jaw length as a proportion of SL significantly differs between the two species. The ranges of the eye horizontal diameter also overlap (5.6−7.8% SL, mean 6.6, in S. janae vs. 4.8− 6.8% SL, mean 5.6, in S. squalus from Po and Soča) but the difference is statistically significant. The eye in S. janae is always larger when specimens of about the same length are compared ( Fig. 4 View FIGURE 4 ). Besides, the difference is significant between the values for the eye horizontal diameter as a proprtion of the interorbital width: in S. janae it averages 60.6% while in S. squalus it varies from 53.6 to 55.8%.
Squalius janae has a pointed snout while in S. squalus it is usually more rounded ( Figs. 4 View FIGURE 4 , 5 View FIGURE 5 ); however, the position of the anteriormost point of the mouth cleft and the lower jaw− quadrate junction is about the same relative to the eye margin as it is seen from Figs. 4a,b View FIGURE 4 , though the proportions and shape of the mouth are different: in S. janae the mouth cleft is longer, straight and oblique, while in S. squalus the mouth cleft is shorter, curved in its anterior part and more horizontal. Besides, the new species has a distinctly projecting upper jaw and no chin at the mandibular symphysis in contrast to S. squalus , with the upper jaw not projecting relative to the lower jaw and a well marked chin. However, shape of the mouth and length of the lower jaw varies to some extent in S. squalus ; among the specimens examined, the sample from the River Nadiža in the Soča drainage is the most divergent ( Fig. 6 View FIGURE 6 ), having a distinctly subterminal mouth with an anteriormost point of the cleft on about the level of the lower margin of the eye and a projecting upper jaw.
The new species is further distinguished from S. squalus by having usually 44 total vertebrae, the vertebral formulae being 24+20 or 25+19 vs. usually 43 (25+18). We examined 18 specimens from the Soča and Osapska and found the following counts: 43 total vertebrae in all specimens, 25 (15) or 24 (3) abdominal vertebrae, 15 (15) or 16 (3) predorsal vertebrae, 18 (15) or 19 (3) caudal vertebrae, vertebral formulae 25+18 (15) or 24+19 (3).
Squalius janae has a silvery colouration in life ( Fig. 3 View FIGURE 3 ) vs. darker colouration with brownish or slight bronze tones in S. squalus ( Fig. 5 View FIGURE 5 ). There is a row of dense pigment black dots along the outer margin of scales on back and flanks forming a regular reticulate pattern in S. squalus ( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 , 8b View FIGURE 8 ) though sometimes pigments are dense on scale pocket forming vertically elongate black spots ( Fig. 5 View FIGURE 5 ) similar to those in S. illyricus ( Bogutskaya & Zupančič 1999, Fig. 5b View FIGURE 5 ). However, pigmentation is never dense and conspicuous in S. janae , and a series of pigment dots along the outer margin of scales is always narrow and sometimes indistinct ( Fig. 8a View FIGURE 8 ), especially in smaller specimens.
We would like to emphasize that we identify a Squalius from rivers flowing to the Adriatic Sea immediately in the north and south from the Dragonja—Osapska Reka, Rižana, an endorheic Malinska ( Fig. 7 View FIGURE 7 ), and Mirna,—as S. squalus . It possesses the most characteristic features of the latter, namely, a short head (the head length is less than 29% SL), a short lower jaw (the lower jaw length is less than or about equal to the caudal peduncle depth), a slightly curved mouth cleft, a comparatively small eye, a dark overall colouration with bronze shades, and dark pectoral, pelvic and anal fins with intensive black pigment in larger juveniles and, especially, adults, with orange to red shades visible to lesser or greater degree depending on the development of black pigment. The presence of red or orange pigments in pectoral, pelvic and anal fins in S. squalus has been mentioned in the literature ( Bruno 1987; Forneris et al. 1990; Gandolfi et al. 1991) and also observed in our specimens, though in larger spawning individuals the fins are very dark, almost black with the red or orange pigments only weakly visible. The pigmentation of the lower fins and the body in S. squalus from Slovenia is also illustrated by Zupančič (2009, six figures on p. 31).
Squalius zrmanjae (Zrmanja and Krka Rivers) and S. janae share such characters as a relatively slender body, 9½ anal-fin branched rays, a shallow head with a conical slightly pointed snout and subterminal mouth, yellow pigment in pectoral, pelvic and anal fins, black pigment on flank scales concentrated in scale pockets forming regular dark spots, and about 43–49 total lateral-line scales. However, S. zrmanjae does not belong to the S. cephalus species group for it has a usually absent free 5 th infraorbital or, if present, never extensive (vs. extensive in the latter), a low number of cephalic sensory pores, usually 11 in the CSO (vs. usually 12–13) with 6–7 openings on the frontal (vs. 7–9), usually 15–19 in the CIO (vs. 18–22), and 4–6 the CPM openings on the dentary (vs. usually 6–8). Besides, S. janae differs from S. zrmanjae in having a long lower jaw, 39– 45% HL, which is longer than the depth of the operculum (vs. short, 34–38% HL, shorter than the depth of the operculum), and a straight oblique mouth cleft (vs. curved and more horizontal).
We do not provide here any comparison with Squalius cf. squalus from the Krka and Neretva drainages for it is an object of a study in preparation. We suspect they may represent distinct species. Squalius sp. is common in the lower and middle course of the Krka (up to Roski Slap), also known from the upper Krka (NMW 49183, 49203, Knin) where it is probably rare. A Squalius is also widely spread in Neretva basin including Jablanicko Lake ( Aganovič et al. 1966), Rama, Buna, Bregava, Krupa rivers including Hutovo Blato and in River Trebišnjica and Bilečko Lake (artificial) in the Eastern Herzegovina, and Bačina lakes in Croatia ( Zupančič 2008).
Squalius illyricus distributed in Cetina and Krka (one syntype is from Isonzo though there is no recent data from this river) and S. janae have a similar colouration—a black spot on scale pocket and a narrow series of melanophores along the outer margin of each flank scale, and the presence of yellow and black pigment on the pectoral, pelvic and anal fins. However, the new species is clearly distinguishable by a fewer total lateralline scales (40–48 vs. 45–55), a pointed snout with a projecting upper jaw (vs. a stout, markedly rounded snout), and a long jaw with its junction with the quadrate at about the vertical though the middle of the eye (vs. short, lower jaw–quadrate junction in front of the anterior margin of the eye).
Squalius svallize from the Neretva, Trebišnjica and Ljuta drainages stands apart from S. janae by having, first of all, usually 9½ dorsal-fin branched rays and (10) 11–13 (14) gill-rakers in contrast to 8½ and 8–10, respectively, in S. janae .
Squalius prespensis from the Prespa Lake belongs to the S. cephalus group as defined above and can be considered the species morphologically closest to S. janae for the two species share such a character, which is considered unique for the genus Squalius by Kottelat and Freyhof (2007), as a long head. Very similar to that in S. janae , the length of the head in S. prespensis is 27–31% SL and exceeds the body depth by a factor of 1.2–1.3 according Kottelat and Freyhof (2008) (28–29% SL in 5 specimens examined by us). Besides this character, the two species are similar in having a shallow body (the body depth at the dorsal-fin origin is within a range of 22–26% SL), a long lower jaw (11–13% SL), a straight mouth cleft, and about 43–46 total lateral line scales. However, S. janae differs by a larger eye (eye horizontal diameter is 53–69% interorbital width vs. 48–50 in S. prespensis ), a pigmentation pattern on flank scales (absence of a regular reticulate pattern, vs. presence), and often 9½ branched anal-fin rays (only 8½ in 5 specimens of S. prespensis examined by us).
We did not examine specimens of the other ten Squalius species from the Aegean and East Adriatic (south from the Skadar and Ohrid) basins, and can use only published data (e.g. Kottelat & Freyhof 2007) for comparison. Squalius janae can be distinguished from all these species by having a long head and a long lower jaw as discussed above, and further from S. vardarensis , S. pamvoticus , S. orpheus , Squalius sp. Aoos, and Squalius sp. Evia by 9½ anal-fin branched rays (vs. usually 8½).
The new species can be also easily distinguished from S. lepidus , S. anatolicus and S. kottelati in having the upper jaw projecting beyond the lower jaw vs. the lower jaw considerably projecting and forming a prominent chin ( Bogutskaya 1994, 1997; Turan et al. 2009). We do not provide here a detailed comparison with Anatolian, Caucasian and Middle East species or populations of the S. cephalus group ( S. orientalis s.l.) for we suppose that the new species is not conspecific with them because of historical and zoogeographical reasons, and also because it differs by some vertebral counts—43−44 total, 15 predorsal and 18−20 caudal vs. usually 39−42, 14 and 16−18, respectively (Bogutskaya, unpublished data)
Discussion of distribution. Squalius janae is distributed in River Dragonja draining westwards in Istra [Istria] which is the northernmost peninsula along the eastern Adriatic coast, positioned between the Gulf of Trieste and the Gulf of Kvarner. The Brkini Region separates Istra from central Slovenia. The peninsula is geologically highly heterogeneous, consisting of alternating limestone and marly sandstone flysch areas. The Dragonja flows along the northern margin of the Istrian Anticline that borders the Trieste Flysch Basin interconnected with the Pazin Flysch Basin in the south. The latter flysch basin is bordered in the north-east and east by the Č ičarja Hills and the Učka Mountain which are largely limestone areas similar to the large northeast-southwest oriented Istrian Anticlines ( Fišer et al. 2006). The historical (paleogeographical) and geological complexity of this relatively small region may be a reason for the narrow endemism of Istrian chub S. janae . The distribution of this species, which is rather similar to that of some groups of aquatic invertebrates (for example, species of Niphargus ( Amphipoda )), can be explained as reflecting its occurrence in a pre-Pleistocene palaeodrainage that disrupted into halves, draining in part towards the northwest, in part towards the southeast, during the Pleistocene karstification of the Dinarides ( Sket 2002; Fišer et al. 2006). It is also worth mentioning that only one further freshwater fish species occurs in Dragonja, Barbus plebejus ( Povz 2002; our observations), while in the adjacent rivers—Isonzo, Mirna, and Pazinčica in Istra, and Zrmanja and Krka in Dalmatia—there are, besides Barbus , also various species of Rutilus , Alburnus, Salmo and Cottus . This supports the idea of substantial historical isolation of the Dragonja drainage. Squalius squalus is also absent from Zrmanja River, which is inhabited by the endemic S. zrmanjae , although both Dragonja and Zrmanja were connected with the Po and Isonzo drainages in the Pleistocene during glacial sea regressions (Rodić 1981).
| SMNH |
Department of Paleozoology, Swedish Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |