Litoria graminea ( Boulenger, 1905 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4457.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:EFBF6BB5-2283-490C-94A8-1F20B742F18A |
|
DOI |
https://doi.org/10.5281/zenodo.5988821 |
|
persistent identifier |
https://treatment.plazi.org/id/293FBB0A-123C-4E69-FF65-FDC27813FABE |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria graminea ( Boulenger, 1905 ) |
| status |
|
Litoria graminea ( Boulenger, 1905)
Fig. 2 View FIGURE 2
Hyla graminea Boulenger, 1905: 183 .
Litoria graminea Tyler 1971: 353 .
Litoria dux Richards & Oliver, 2006: 50 .
Nyctimystes dux Duellman, Anstis & Hedges 2016: 39 View in CoL .
Dryopsophus gramineus Duellman, Anstis & Hedges 2016: 40 View in CoL .
Ranoidea graminea Dubois & Frétey 2016: 21 .
Material examined for rediagnosis. BMNH 1947.2 .23.32 [originally 1905.1.30.29], holotype, “N coast of Brit. New Guinea, 900 ft.” ; BMNH 1935.3 .9.203, Kokoda , Northern Province, Papua New Guinea ; BPBM 1074 About BPBM , Kapara-Sengi , near Kokoda, 8.9330° S, 147.8038° E, 600 m, Northern Province, Papua New Guinea GoogleMaps ; BPBM 35405–08 About BPBM , Jarefa Camp , near Itokama, 9.2053° S, 148.2375° E, 820 m, Northern Province, Papua New Guinea GoogleMaps ; BPBM 25847–51 About BPBM , 25854 About BPBM , 31428–30 About BPBM , 31805 About BPBM , 1.3 About BPBM km N and 6.2 km W of Cape Dinga, Kamiali Wildlife Management Area , 7.2960° S, 147.0929° E, 520 m, Morobe Province, Papua New Guinea GoogleMaps ; BPBM 25852–53 About BPBM , 2.6 About BPBM km N and 9.3 km W of Cape Dinga, Kamiali Wildlife Management Area , 7.2819° S, 147.0650° E, 900 m, Morobe Province, Papua New Guinea GoogleMaps .
Diagnosis. Litoria graminea is distinguished from all congeners in possessing the following unique combination of characters: large size (male SV = 67.0– 76.4 mm, female SV = 50.4–71.9 mm); moderately long leg (TL/SV = 0.53–0.60); relatively broad snout (EN/IN = 1.00–1.32); relatively wide head (HW/SV = 0.34–0.40); absence of parotoid glands; a reticulum of deep creases in skin of shoulder region, these creases often lined in white or pale yellow; fully webbed hands; small round or oblong nuptial pads occupying an area approximately equal to one-quarter to one-half area of first finger disc; dorsal color bright green in life (blue in preservative); white stripe on lower jaw extends no farther posteriorly than posterior margin of eye; iris tan or pale gray with a reddish outer margin, finely stippled with black; pale portion of sclera typically 3–6 times width of black margin separating sclera from cornea; upper half of nictitating membrane clear except for a dark dorsal margin; dorsal surface of thighs green in life, blue in preservative; and groin, hidden surfaces of limbs, and webbing of hands and feet orange or yellow in life.
Comparisons with other species. Litoria graminea differs from all other named Papuan Litoria in its combination of large body size; bright green dorsal coloration; fully webbed hands; a reticulum of deep creases in skin of shoulder region; absence of enlarged parotoid glands; upper half of nictitating membrane clear except for a dark dorsal margin; and iris tan or gray with a reddish outer margin in life, finely stippled with black. As noted above, the reticulum of skin creases in the scapular region appears unique among Papuan Litoria .
Description. Head wide (HW/SV = 0.34–0.40), wider than long (HL/SV = 0.31–0.39, HL/HW = 0.86–0.98); loreal region oblique and concave; canthus rostralis concave, rounded; nostrils oriented laterally, closer to tip of snout than to eyes; internarial distance usually less than distance from external naris to eye (EN/IN = 1.00–1.32, IN/SV = 0.076–0.095, EN/SV = 0.081–0.111) but of equal length in one specimen; snout truncate to slightly sloping when viewed from the side, broadly rounded when viewed from above; eyes moderate (EY/SV = 0.089– 0.122), not protuberant, eyelid less than half width of interorbital distance; tympanic ring distinct and raised but top margin covered by supratympanic skin fold, horizontal diameter half to three-quarters as large as eye (TY/EY = 0.51–0.78) ( Fig. 2 View FIGURE 2 ).
Skin of dorsal surfaces smooth, with narrow creases in scapular region, laterally, and around vent, these often margined in white or pale yellow; ventral surfaces of body and thighs coarsely granular, less so on chin and under arms, smooth under shanks. A pale dermal ridge extends along the outer surface of each forearm and along each foot from the heel to T5. Peritoneum white.
Fingers fully webbed between F2, F3, and F4, and half webbed between F1 and F2, with webbing reaching to discs of F2 and F4 but falling slightly short of discs on F1 and F3; relative lengths 3> 4> 2> 1. Tips of all fingers flattened into discs bearing circum-marginal grooves; discs approximately 1.5 times wider than penultimate phalanges on F2–F4 but only slightly wider than penultimate phalanx on F1; single subarticular tubercle present at base of each penultimate phalanx; thick inner and low outer metacarpal tubercles present. Nuptial pad of darkbrown dermal asperities a small circle or small oblong occupying an area approximately equal to half or less that of the disc of F1. Toes fully webbed, webbing reaching discs of all toes; relative lengths 4> 5> 3> 2> 1; tips flattened into discs with circum-marginal grooves; discs approximately 1.5 times width of penultimate phalanges; inner metatarsal tubercle prominent, outer not evident. Hind legs moderately long (TL/SV = 0.53–0.60).
Vomeropalatines with two patches of teeth between internal nares. Vocal slits and sac present.
In preservative, dorsal ground color varies from uniform light blue to dark blue gray, as do the tops of thighs, shanks, feet, forearms, F4, and T5. Forearms white with light dusting of gray stippling evident under magnification; tops of outer fingers, toes, and intervening webbing typically dusted with gray. Venter uniformly white; remainder of digits and webbing white. Color of tympanum same as adjacent skin; a translucent horseshoeshaped figure, which is usually dark but may be pale, often present on the tympanum. Margins of eyelids and external nares usually white. White stripe present on dermal ridges of forearm and foot. Lower jaw white from symphysis to below eye; behind this, lower jaw blue. Upper half of nictitating membrane clear with a dark upper margin. Iris tan dusted with black punctations. Sclera white, with a narrow black margin where it contacts cornea; white area of sclera usually 3–6 times width of this black corneal rim.
Color in life. Dorsal ground color uniformly bright green, with anterolateral reticulum of creases often margined in white or yellow, white labial stripe extending no farther posteriorly than posterior margin of eye, the dermal ridges along outer margins of legs and arms usually white but may be yellow. Sides yellow; ventral surfaces yellow on chin and chest, white on abdomen; undersides of forearms, shanks, and front of thighs pale blue gray or orange; groin, axilla, and rear of thighs bright orange, with flecks of bright orange extending down undersides of thighs and shanks. Tops of outer digits green, of inner digits yellow; webbing of hands and feet bright orange. Iris tan or pale gray, typically with a reddish outer margin, minutely suffused with black. Black corneal rim at junction with sclera; sclera pale blue-white.
Variation. The range of mensural variation is not great ( Table 1). Extent of webbing on the hands and feet is also remarkably uniform. There is important variation in the extent of the scapular and lateral skin creasing. These creases are deep, extensive, and margined with white in the holotype (BMNH 1947.2.23.31), margined in white in BMNH 1935.3.9.203 from Kokoda, and margined in yellow in BPBM 1074 from near Kokoda, Northern Province. These creases are present in all specimens from Itokama, Northern Province, and Kamiali, Morobe Province, but the depth of furrowing and the extent of light margining varies, making the conspicuousness of the creases variable. The Itokama specimens generally have deeper creases than do the Kamiali specimens, and three of the four Itokama specimens have the creases with pale margins, but only four of 12 specimens from Kamiali are so outlined. Hence, it may be that this reticulum of creases is better developed in the south (Kokoda, Itokama) than in the north (Kamiali), suggesting the possibility of clinal variation.
The tops of the thighs are always blue (green in life) but in one specimen (BPBM 35408) this color field is reduced to a thick line, whereas it is more extensive in all other specimens. The white venter and white lines on the dermal ridges of forearms and feet are invariant. One specimen from Itokama (BPBM 35408) has the white margin to the eyelid reduced in width and contrast with the surrounding skin. The white labial stripe extends posteriorly from the symphysis to a point ranging anywhere from anterior to the eye to the posterior margin of the eye. The skin over the tympanum is well-developed, and often, though not always, has a horseshoe-shaped translucent area.
All specimens have a pale tan or pale gray iris stippled with black; in life, the iris is typically tan centrally grading to reddish towards the outer margin. The sclera of all specimens consists of a narrow margin of black pigment at its junction with the cornea, outside of which is a field of white (pale blue in life) before it joins to the eyelid. But the width of this pale scleral field between the black corneal margin and the eyelid varies considerably. In most specimens this pale band is broad, varying from 3–6 times the width of the black circum-marginal band around the iris. But in some specimens, this pale scleral field is reduced to a narrow strip approximately equal to the width of the black rim (e.g., BPBM 25854, 35405, 35408) or is entirely absent (e.g., BPBM 31428). The pale portion of the sclera tends to be narrower in the Itokama sample than in the Kamiali specimens. All specimens have the top half of the nictitating membrane clear with a dark upper margin; the bottom half in all specimens is heavily spotted with dark pigment.
Etymology. The name is a feminine Latin adjective meaning “grassy” and is no doubt in reference to the species’ green coloration.
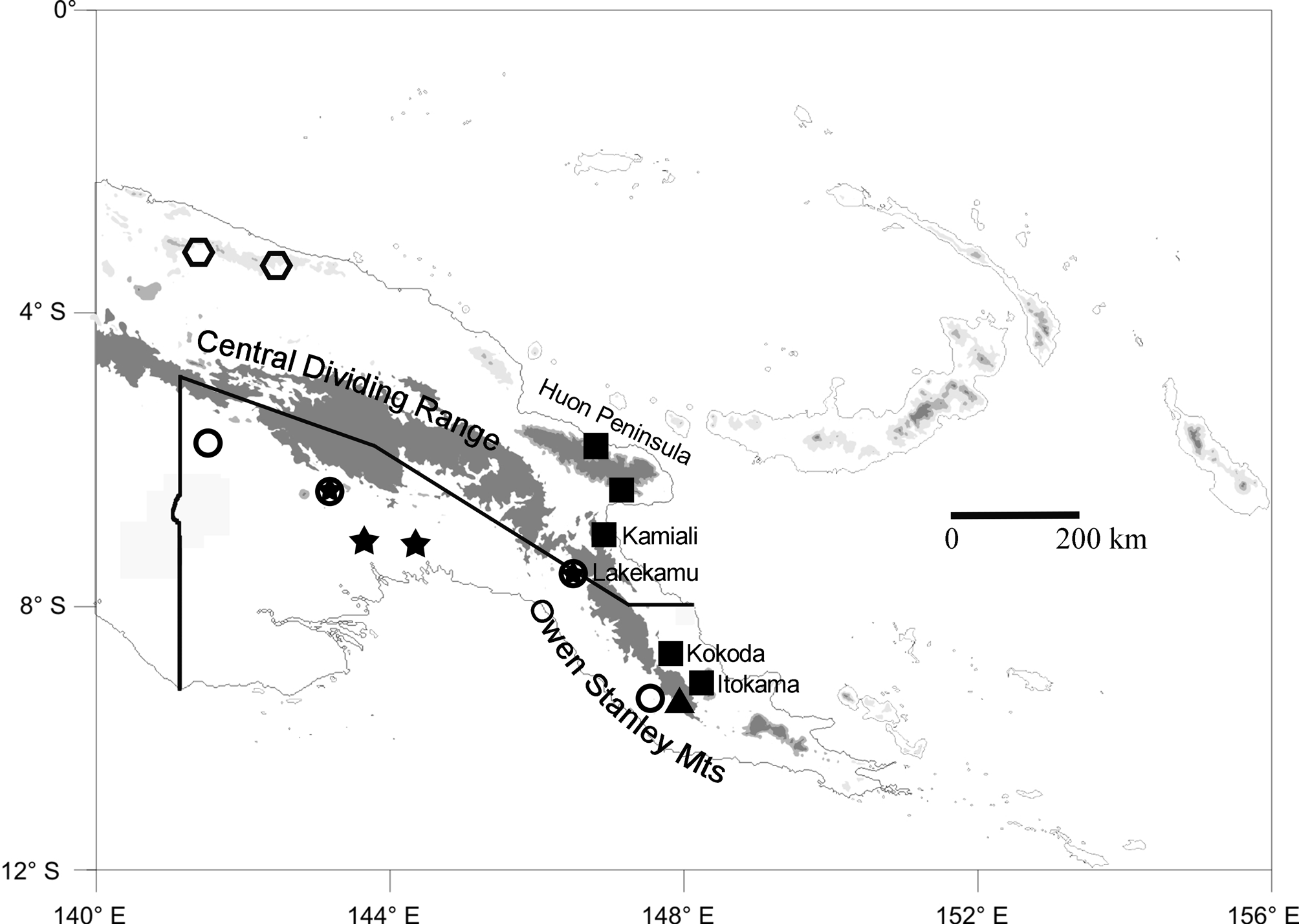
Range. Known from the northern versant of the Owen Stanley Mts, Northern Province, and the Huon Peninsula of Morobe Province, Papua New Guinea ( Fig. 1 View FIGURE 1 , squares).
Remarks. Prior diagnoses of Litoria graminea have suffered from small sample sizes, mis-assignment of the type locality, and/or confounding of multiple species in the samples studied. The sample used for the diagnosis above is based on 19 individuals from three areas, all from the geographic region from which the holotype was collected. Boulenger’s (1905) original description was based on only a single animal; Tyler (1968) examined three specimens, including the holotype, but two of those were from far-western New Guinea and likely represent a different species; and, as discussed above, Richards & Oliver (2006) examined only two specimens, including the holotype, but the second of these represents the species described next. Only the holotype of L. graminea has accurately represented that species in these prior publications. Parker (1936) had available two specimens of true L. graminea but had no need for a rediagnosis or redescription at that early date.
Richards & Oliver (2006) introduced several features (summarized in their table 2) that they argued were useful for differentiating their “ Litoria graminea ” from the species L. sauroni and L. dux , newly named by them. The variation seen in my sample of L. graminea makes clear that several of these are not diagnostic. My measurements for SV, TL/SV, HW/SV, and HW/HL within L. graminea either span the entire range of variation reported for their samples of L. sauroni and L. dux (SV, TL/SV), or so greatly overlap the range of variation seen (HW/SV = 0.33–0.36 in L. sauroni vs. 0.34–0.40 in my sample of L. graminea ) as to render the morphometric measurements non-diagnostic. I have measured all three type specimens of L. dux and am unable to repeat their claim of a measurement of 1.3 for HW/HL (I measured HW/HL = 1.03–1.10 for the specimens). Dividing their maximal reported value for HW (24.1 mm) by their minimal reported value for HL (22.1 mm) gives a value of 1.09, suggesting that the value of 1.3 given in their table 2 is a typographical error; hence, my sample of L. graminea provides complete overlap with both species in that feature as well. Thus, none of these features will serve to diagnose these species.
Some of the color-pattern features used by Richards & Oliver (2006) are also not useful for diagnosis but may have initially appeared so because of small sample sizes. The white margin to the upper eyelid varies in thickness and intensity in my samples, being virtually absent in one of my specimens. And the ventral coloration in life listed for L. graminea in table 2 of Richards & Oliver (2006) is instead based on a specimen of L. sauroni , as discussed above.
More complicated is the claim that scleral coloration is diagnostic, with Richards & Oliver (2006) arguing that the sclera was blue in living L. graminea and L. sauroni but black in L. dux . In all L. graminea specimens I examined, the boundary between the cornea and sclera is a narrow black rim, with the sclera proximal to that being pale blue or blue-white in most specimens. But this blue scleral field varies considerably in width (both between specimens and around the corneal margin in the same eye) and is not obvious in a few, making it appear to superficial examination that the “sclera is black”. What accounts for this variation in uncertain, but it suggests that this presumptive difference is an artifact, especially if determination of the character was based on photographs of living animals instead of direct examination of preserved specimens, which is how Richards & Oliver (2006) made their sclera-color determinations (P. Oliver, pers. comm.). But that is an unreliable means for determining this feature inasmuch as the black border between the cornea and sclera is present in all animals and the surrounding blue sclera is not obvious in living animals in which the eye is not fully protrusive. For example, this is true in photographs taken in life of BPBM 35407, in which the blue sclera is not evident in some photographs, but is nonetheless present in others and is obvious in the preserved specimen. Proper determination of whether the sclera of these frogs is truly black requires direct examination under a microscope by lifting the upper eyelid. I have seen no specimens clearly possessing a black sclera inasmuch as the specimens of L. graminea I have seen lacking the blue sclera have the upper eyelid contacting the eye directly at the black corneal-scleral boundary instead of distal to that point, as is usual in most specimens. It is unclear whether this variable connection to the upper eyelid is due to some form of preservation variation or not, but it seems reasonable to treat the claim that scleral coloration is diagnostic in these frogs with skepticism, and I conclude that the character is non-diagnostic pending a more compelling demonstration of its utility. However, the width of the blue portion of the sclera may be diagnostic for the next species described relative to the general averages seen in other species ( Table 2).
This leaves us with three characters introduced by Richards & Oliver (2006) that do serve to distinguish among members of the Litoria graminea species-group: iris pattern, nictitating membrane pigmentation, and size and shape of the nuptial pads ( Table 2). Again, however, the character states claimed by Richards & Oliver (2006) to apply to L. graminea for the first and last of these are incorrect. Litoria graminea has a pale tan or pale gray iris with a reddish outer ring, and the nuptial pads are small and round or oval (an observation also made by Parker [1936]). Thus, there are currently no reliable differences that serve to distinguish L. dux from true L. graminea : both taxa have the same iris color pattern, the same pigmentation pattern on the nictitating membrane, the same form of the nuptial pads, and the same limited extent to the white labial stripe (important for distinguishing from the species described next). These nominal taxa do appear to differ in a few elements of color pattern in life. To wit, the extensive reticulum of anterolateral creases seen in southern specimens of L. graminea is not evident in the photograph of L. dux provided by Richards & Oliver (2006). However, skin creasing is apparent in photographs I have taken of the three type specimens of L. dux . As noted earlier, this creasing appears to vary between populations, perhaps clinally, so its poor development in northern animals cannot alone be taken as evidence of species differences. Second, Richards & Oliver (2006) note that the venter, webbing, and hidden surfaces of the limbs are all yellow in living L. dux . In samples of L. graminea from farther south each of these areas is bright orange. However, Parker (1936) noted that his specimen from Kokoda (BMNH 1935.3.9.203) had the hidden surfaces of the limbs bright yellow in life. Animals from Kokoda (and throughout that more southern region of New Guinea) are clearly conspecific with the holotype of L. graminea , judging from the presence of the diagnostic skin creasing in the scapular region, small round nuptial pad, tan iris lacking dark flecks, and clear upper nictitating membrane. Hence, it appears that L. graminea is variable in the coloration of the hidden surfaces of its limbs, obviating this feature as diagnostic for L. dux . Further, the closely related L. sauroni shows considerable variation in coloration of these areas, including various combinations of cream, yellow, orange, purple, and purple-blue (Richards & Oliver 2006), so intraspecific variation in L. graminea that included yellow and orange could not be viewed as exceptional in this complex. Present evidence indicates that L. dux is a junior synonym of L. graminea , given the variable (and apparently clinal) nature of the anterolateral creasing, variable color of the hidden surfaces of the limbs in L. graminea , doubtful utility of purported differences in scleral coloration, and the absence of differences in features proven to be diagnostic in this species-group (e.g., iris color pattern, nictitating membrane pigmentation pattern, nuptial pads, body proportions).
Following this synonymization, there are now two valid names that have been applied to members of the Litoria graminea species-group: L. graminea ( Boulenger, 1905) , and L. sauroni Richards & Oliver 2006 . This species-group can be defined by the unique combination of large size, horizontal pupils, no parotoid glands, and fully webbed hands. Litoria graminea has been rediagnosed above; L. sauroni is differentiated from that species by its red or dark-orange iris extensively flecked/reticulated with black (vs. tan with a reddish margin, suffused with black stipples in L. graminea ), nictitating membrane pigmented with a dark upper margin and dark flecking below this (vs. clear below the dark upper margin in L. graminea ), absence of scapular creasing in the skin (present to some degree in L. graminea , and conspicuously developed in southern populations), and frequent presence of purple on the ventral and hidden surfaces of limbs (vs. never present in L. graminea ). Litoria sauroni occurs at lower elevations along the southern versant of the Central Dividing Range of Papua New Guinea ( Fig. 1 View FIGURE 1 ).
The recently described L. huntorum was not originally assigned to the L. graminea group (Richards et al. 2006) but seems to belong there on the basis of its relatively large size, fully webbed hands, absence of paratoid glands, and bright-green dorsal coloration. It differs from both L. graminea and L. sauroni in its smaller adult size (male SV = 57.9–60.4 mm), in having two nuptial pads on each first finger, in having a white labial stripe that extends posterior to the eye, and in its longer call (0.7– 0.9 s vs. <0.5 s). It is known from two north-coast mountain ranges in northwestern Papua New Guinea ( Fig. 1 View FIGURE 1 ).
This leaves two additional species to be described that I have obtained during the course of herpetofaunal surveys in Papua New Guinea. The first of these has also been present for years in several museum collections but has gone unnoticed.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Litoria graminea ( Boulenger, 1905 )
| Kraus, Fred 2018 |
Hyla graminea
| Boulenger, 1905 : 183 |
Litoria graminea
| Tyler 1971 : 353 |
Nyctimystes dux Duellman, Anstis & Hedges 2016 : 39
| Hedges 2016 : 39 |
Dryopsophus gramineus Duellman, Anstis & Hedges 2016 : 40
| Hedges 2016 : 40 |
Ranoidea graminea Dubois & Frétey 2016 : 21
| Dubois & Frétey 2016 : 21 |