Russula pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck, 2023
|
publication ID |
https://doi.org/ 10.5252/cryptogamie-mycologie2023v44a3 |
|
DOI |
https://doi.org/10.5281/zenodo.7829750 |
|
persistent identifier |
https://treatment.plazi.org/id/255E7B23-FFC4-FFD4-C966-8FB1646DFCFB |
|
treatment provided by |
Felipe |
|
scientific name |
Russula pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck |
| status |
sp. nov. |
Russula pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov.
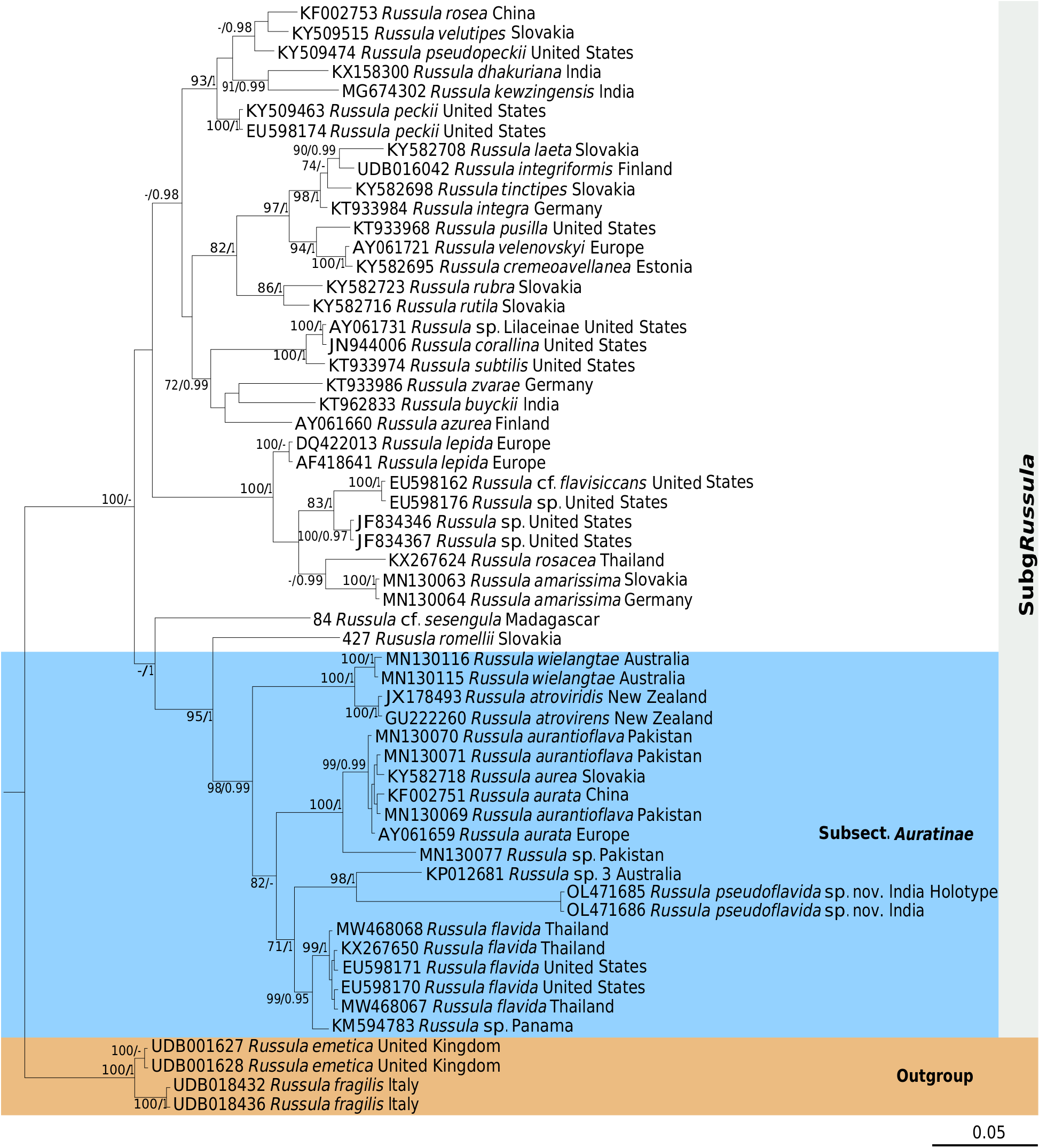
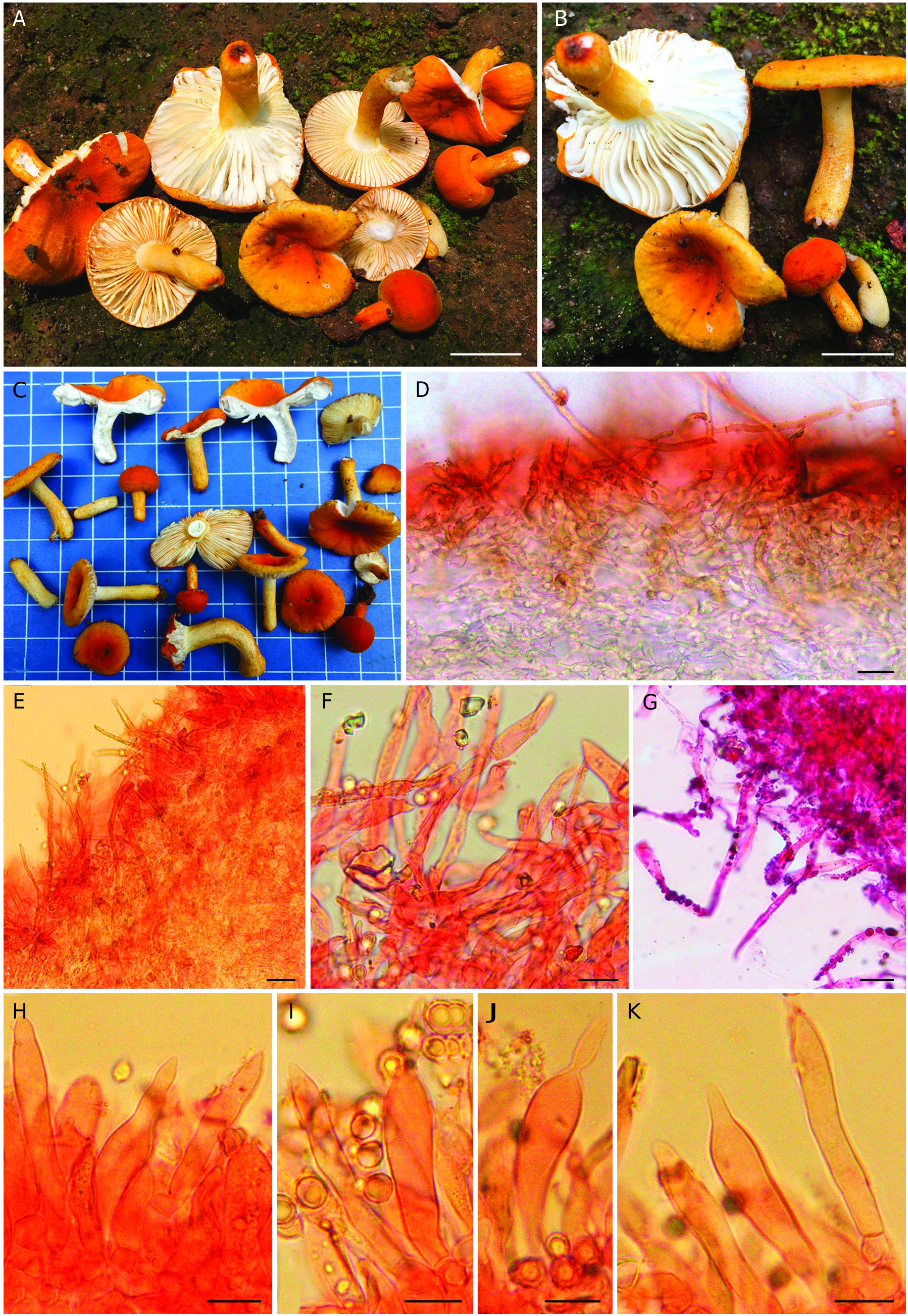
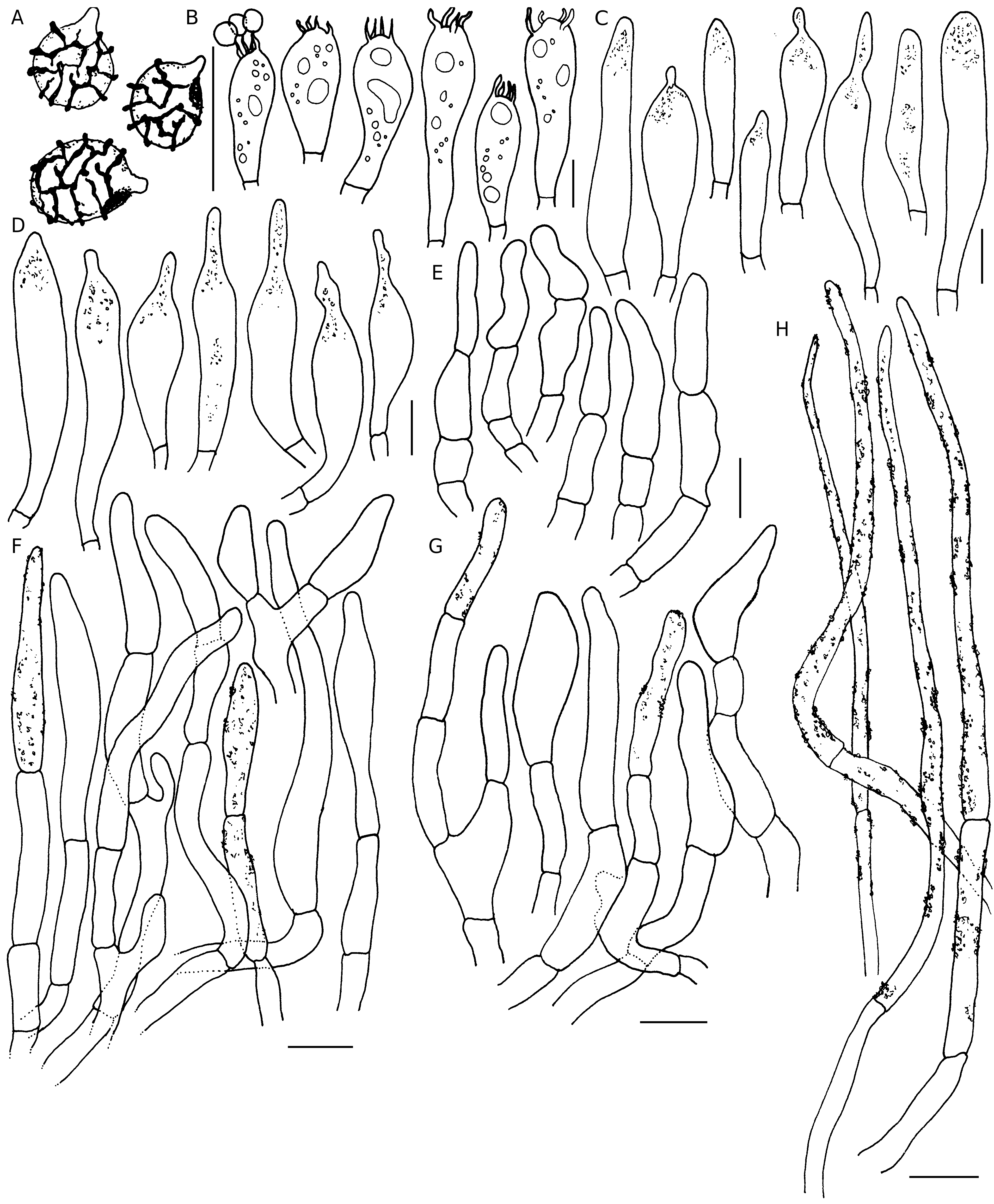
( Figs 7-9 View FIG View FIG View FIG )
Russula pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov. differs from North American R. flavida Frost ex Peck in its very small to medium sized (10-45 mm) pileus, very long primordial hyphae usually with strong incrustations covering most of the surface, distinctly smaller spores and occurrence under Shorea robusta .
HOLOTYPE. — India. West Bengal, Jhargram district, Tuluha , 22°19’18”N, 87°05’34”E, alt. 80 m a.s.l., on ground, under Shorea robusta in tropical deciduous forests, 13.VIII.2020, A. Ghosh, AG 20-058 (holo-, CAL [ CAL 1862 ]!). GoogleMaps
ADDITIONAL SPECIMENS EXAMINED. — India. West Bengal, Paschim Medinipur district, Chandra , 22°21’01”N, 87°02’00”E, alt. 90 m a.s.l., on ground, under Shorea robusta in tropical deciduous forests, 12.VIII.2020, A. Ghosh, AG 20-022; GoogleMaps Jhargram district, Lodhasuli , 22°19’50”N, 87°01’41”E, alt. 80 m a.s.l., on ground, under S. robusta in tropical deciduous forests, 13.VIII.2020, A. Ghosh, AG 20-036; GoogleMaps Jhargram district, Jhargram city, 22°25’01.1”N, 87°00’13.5”E, alt. 103 m a.s.l., on ground, under S. robusta in tropical deciduous forests, 12.VIII.2021, A. Ghosh, AG 21-070 ( CAL [ CAL 1863 ]); GoogleMaps Bihar , West Champaran district, Valmiki national Park , Raghia range, Sitalbari enclosure, 27°20’14.4”N, 84°13’05.8”E, alt. 133 m a.s.l., on ground, under S. robusta in tropical deciduous forests, 15.IX.2020, M.E. Hembrom, MEH-20-110; GoogleMaps Jharkhand, Rajmahal hills, Sahibganj district, Borio block, Pir-Baba Kairasol forest area, 25°09’41.7”N, 87°40’31.9”E, alt. 126 m a.s.l., on ground, under S. robusta in tropical deciduous forests, 24.VIII.2021, M.E. Hembrom, MEH-21-06; GoogleMaps Rajmahal hills, Pakur district, Hiranpur block, Talpahari to Tugutola forest area, 24°37’02.6”N, 87°40’45.2”E, alt. 94 m a.s.l., on ground, under S. robusta in tropical deciduous forests, 26.VIII.2021, A. Ghosh, AG 21-11 ( JH). GoogleMaps
GENBANK. — OL471685 (nrITS, holotype) and OL471686 (nrITS, specimen voucher no. AG 21-070); ON365928 (nrLSU, holotype), ON365929 (nrLSU, specimen voucher no. AG 21-070); ON387512 (mtSSU, holotype), ON387511 (mtSSU, specimen voucher no. AG 21-070); ON398067 (rpb 2, holotype), ON398068 (rpb 2, specimen voucher no. AG 21-070).
ETYMOLOGY. — Referring to its being a look-alike and close relative of R. flavida , a North American species in the crown clade of Russula subg. Russula .
MYCOBANK. — MB 844206.
FACESOFFUNGI NUMBER. — FoF 11437.
DESCRIPTION
Pileus very small to medium-sized, 10-45 mm in diam., convex when young, becoming planoconvex to applanate, uplifted with age, centrally depressed to umbilicate with maturity, margin tuberculate striate, decurved to plane with age; cuticle smooth, velvety, viscid and shiny when wet, dull upon drying, peeling to 1/2 of the radius, deep orange (6A-B7-8) or brownish orange (6-7C7-8) when young, then yellowish orange, orange yellow to deep yellow (4A7-8) or even orange to deep orange (5A7-8). Pileus context 5-10 mm thick at the disc, thinning towards the margin, brittle, chalky white (1-2A1), unchanging after bruising or cutting; turning salmon pink (6A4) with FeSO 4 and deep to dark turquoise (24E-F7-8) in guaiacol. Lamellae equal, 10-15 mm high, adnexed to narrowly adnate, normally spaced (10/cm) to crowded (up to 22/cm at pileus margin), rounded near pileus margin, chalky white (1-2A1), sometimes forked near stipe apex; edges even, marginate, deep orange or dark orange (5A8). Stipe 10-30× 4-9 mm, cylindrical, central, firm, with dry, smooth, velvety surface that is concolorous to pileus, but chalky white (1-2A1) at the stipe apex, unchanging after bruising or cutting, turning salmon pink (6A4) with FeSO 4 and deep to dark turquoise (24E-F7-8) in guaiacol, stuffed and chalky white (1-2A1) inside, unchanging. Odour not distinctive.Taste mild. Spore print not obtained.
Basidiospores globose, broadly ellipsoid to ellipsoid, (5.5-) 5.7-6.05-6.5(-7.0)×(4.4-)4.8-5.2-5.6(-6.2) µm, Q=(1-)1.11- 1.17-1.22(-1.25); ornamentation amyloid, composed of obtuse and relatively densely spaced warts, up to 0.6 µm high, merged in short ridges which are interconnected by numerous fine line connections; suprahilar spot amyloid, relatively large and conspicuous; apiculi up to 0.9 µm high. Basidia (18-)21- 27-32(-39) ×(9-)9-10-10.5(-11) µm, 4-spored, subclavate to clavate, sterigmata up to 5 µm long. Hymenial gloeocystidia on lamellae sides (39-)41.5-49-56(-60) × (7-)8-9.5-11(-12) µm, rare, clavate to subclavate and mostly rostrate at the tip (up to 13 µm long), others with narrowing or obtuse-rounded apex, emergent up to 15 µm above the other elements of the hymenium, few deeply embedded; near the lamellae edges usually smaller and narrower, measuring (27-)30-38.5-46.5 (-52) × (6-)6.5-8.5-10(-11) µm; all hymenial cystidia with scarce, granulose contents that do not react in sulfovanillin. Subhymenium layer up to 25 µm thick, pseudoparenchymatous. Marginal cells similar to hyphal terminations in pileipellis, mainly cylindrical, measuring (12-)15-22.5-29.5 (-35) ×(3.5-)4-5-6(-6) µm, apically obtuse-rounded; mixed with occasional basidia or basidioles. Hymenophoral trama mainly composed of large nests of sphaerocytes and intermixed with hyphal elements. Pileipellis orthochromatic in Cresyl blue, sharply delimited from the underlying sphaerocytes of the context, 100-200 µm deep, two-layered; vaguely divided in a 70-150 µm deep suprapellis a trichoderm composed of relatively dense, erect or ascending hyphal terminations; subpellis 30-50 µm deep, composed of more horizontally oriented, densely arranged hyphae. Acidoresistant incrustations uncertain. Hyphal terminations near the pileus margin flexuous, thin-walled, two- to three-celled, branched at the subterminal cells or the cells just below, pigment incrustations abundant; terminal cells measuring (16-)21.5-35-48.5 (-66) ×(4-)5-6.5-8(-9.5) µm, cylindrical or slightly narrowed towards apex or ventricose or narrowly uniform, apically obtuse-rounded or acute; subterminal cells usually equally long but sometimes wider (up to 11 µm), often with lateral projections. Hyphal terminations near the pileus centre of similar structure; terminal cells slightly shorter and less wide, measuring (14-)19-28-37(-45) × (3-)3.5-5-6.5(-9) µm, cylindrical or slightly narrowed towards apex or ventricose or narrowly uniform, apically obtuse-rounded or acute; subterminal cells usually equally long but sometimes wider (up to 13 µm). Potential primordial hyphae near the pileus margin typically 2- to 3-celled, flexuous, very long, thick-walled (up to 1 µm); terminal cells (58-)65.5-111-157(-225) × (2-)2.8-3.8-4.8 (-6) µm, mainly attenuate, apically mostly acute, subterminal cells long, cylindrical; usually with strong incrustations covering most of its surface. Potential primordial hyphae near the pileus centre 2- to 3-celled, flexuous, very long, thick-walled (up to 1 µm), slightly shorter, terminal cells (45-)46-80.6- 115(-165) ×(2-)3.5-4.5-5.5(-6) µm, cylindrical to attenuate, apically mostly acute; usually with strong incrustations covering most of its surface. Pileocystidia not observed. Clamp connections absent in all parts.
NOTES
In the field, our new species is a look-alike of the American R. flavida Frost. It differs microscopically from this American species in the smaller size of its basidiospores, as basidiospores of R. flavida holotype measure (7.1-)7.6-7.9-8.3(-8.6)×(5.8-) 6-6.4-6.7(-7) µm ( Adamčík et al. 2018), while their size was reported as 5.5-8.5(9.6) × 5-7 µm in Bills & Miller (1984) based on different collections.
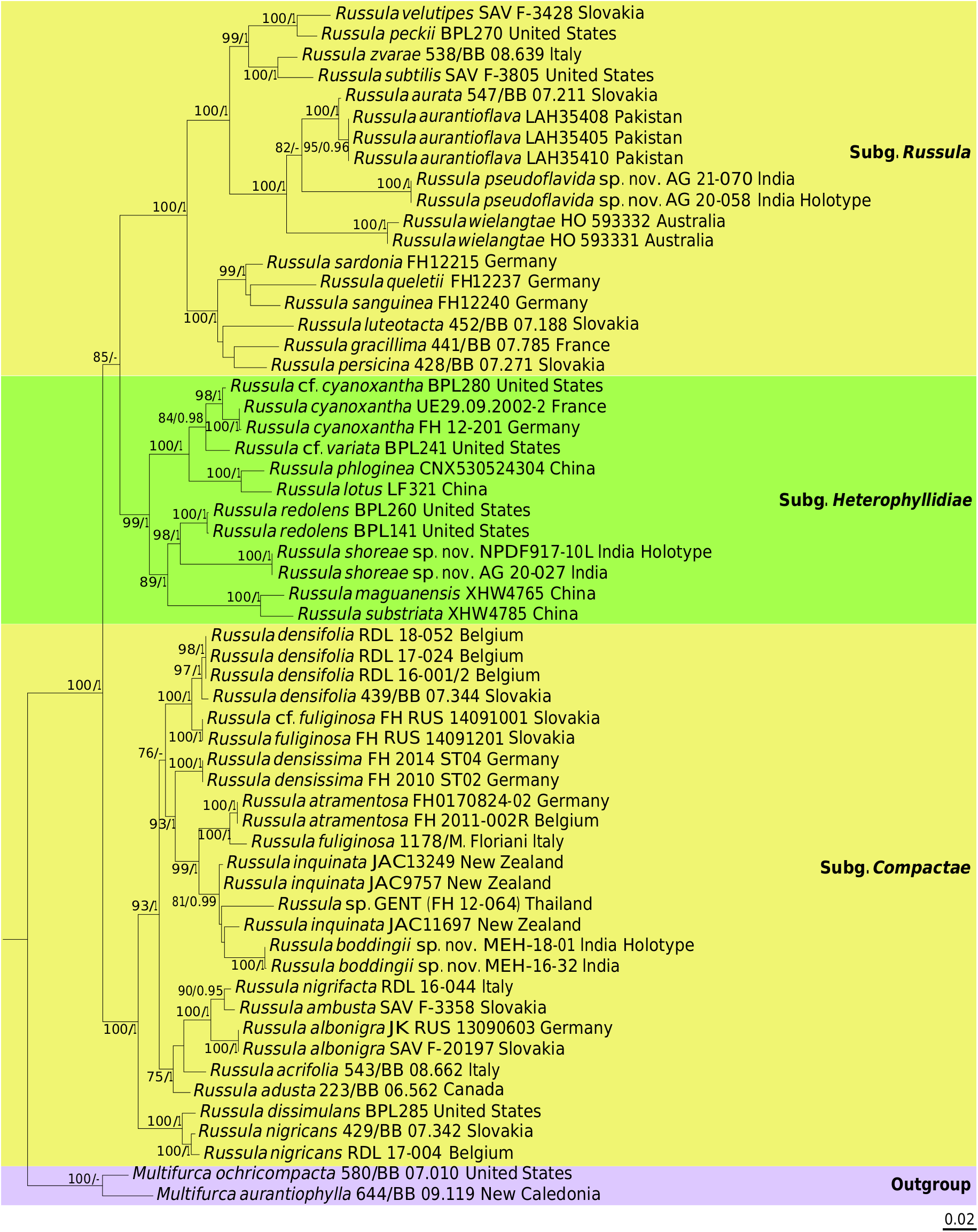
The American R. flavida has not yet been placed in a multilocus phylogeny as essentially ITS sequences are available for this species. Our new R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov. is here placed for the first time on the basis of three genes ( Fig. 3 View FIG ). This placement supports the assumption made on the basis of an ITS phylogeny ( Adamčík et al. 2019) that R. flavida , and now by extension also R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov., might be considered members of subsect. Auratinae Bon. This small subsection was until now limited to merely three species: the European R. aurea and its morphologically and genetically (4 bp difference in the ITS) very similar Asian counterpart, R. aurantioflava , recently reported from Pakistan ( Adamčík et al. 2019), as well as the equally very similar, but rare American R. xantho Shaffer which has not yet been sequenced. Compared to R. flavida and R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov., these species are less uniform in colour with a pileus that varies from purplish to wine red, over brick red and orange to yellow, and a stipe that is frequently tinged with yellow but which can also be entirely white. Additionally, R. xantho is particular in the greying-blackening reaction of the context ( Buyck 2005). The high support obtained in our multigene phylogenetic analyses ( Fig. 3 View FIG ; MLbs= 100%, BPP = 1) and ITS ( Fig. 7 View FIG ; MLbs= 98%, BPP= 1) also suggests that the / wielangtae-lineage should be considered part of Auratinae. This Oceanian lineage, comprising again very few species, the orange-red R. wielangtae from Australia and purplish-greenish R. atroviridis Buyck from New Zealand, offers a very similar microscopy as R. aurea and allies.
When blasting the ITS sequence (which is of perfect quality) of R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov. against GenBank deposits, including environmental sequences, it is immediately evident that this sequence is very different from any other deposited sequence. For nearly complete coverage (100-93%), the closest match is a single Australian sequence at 85.85% similarity, and then similarity drops to less than 83% with first sequences for R. flavida and Auratinae arriving only at 81% similarity; coverage then drops very quickly to 70-60%. This is probably the reason why some of the closer species in multigene phylogenies ( Buyck et al. 2018; Adamčík et al. 2019), such as the European R. romellii or the R. wielangtae lineage don’t show up in these nBLAST results. When doing nBLAST of the ITS of R. romellii , neither R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov. nor Auratinae are showing up in the first 100 results, but R. flavida is at 86% similarity for full coverage.
Host specificity seems not very high for species in Auratinae. The well-documented R. aurea has a distribution that extends from Mediterranean climates all the way into the colder parts of Europe. It occurs under various deciduous trees and conifers, and on various types of soil ( Sarnari 2005). On the other side of the Atlantic Ocean, R. flavida is found in mixed forests with various Quercus , Betula , but also conifers ( Bills & Miller 1984). Russula aurantioflava was originally reported as ectomycorrhizal with conifers ( Adamčík et al. 2019). However, based on 100% similarity top scores in nBLAST for ITS sequence deposits MN704814 and MN 704815 in GenBank, it occurs also in the very north-eastern part of China ( Xing et al. 2020) in forests dominated (98%) by Quercus mongolica with intrusion (2%) of Betula platyphylla Sukaczev , resulting finally in a very similar host range as for both other species. Russula xantho is for the moment the only species of the subsection that seems to have a distinct preference for beech ( Buyck 2005). Our new R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov. is the first species in this lineage that associates with tropical dipterocarps.
The pileipellis of Auratinae has always been interpreted as devoid of any well-defined pileocystidia or primordial hyphae, but they have well-differentiated caulocystidia. However, for R. flavida and R. pseudoflavida A.Ghosh, Hembrom, I.Bera & Buyck , sp. nov., the question of absence/presence of primordial hyphae is more difficult to answer as the entire pileipellis is covered in yellow incrustations and many cells also present deposits inside hyphal terminations. Adamčík et al. (2019) mentioned presence of pileocystidia in the pileipellis of the R. flavida holotype, but absence of primordial hyphae. In our opinion, both primordial hyphae and dermatocystidia are absent in the pileipellis and on the stipe surface, although we admit that the reaction in carbolfuchsine ( Fig. 8H View FIG ) is open for interpretation as most of the colouration is situated inside the hyphae but with some guttules nevertheless sitting on top of the hyphal surface. All of the abovementioned species have also very poor contents in hymenial cystidia.
| CAL |
Botanical Survey of India |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |