Salmoneus degravei, Anker, 2010
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2372.1.18 |
|
persistent identifier |
https://treatment.plazi.org/id/2554563E-FF8C-FB08-A780-FCCE043FF9AF |
|
treatment provided by |
Felipe |
|
scientific name |
Salmoneus degravei |
| status |
sp. nov. |
Salmoneus degravei View in CoL sp. nov.
( Figs. 7–9 View FIGURE 7 View FIGURE 8 View FIGURE 9 , 14c View FIGURE 14 )
Type material. Holotype: breeding female (cl 5.6 mm, tl 15.0 mm), USNM 1124213 About USNM , Panama, Caribbean coast, Isla Grande, southern shore, about 100 m east of western point and Hotel Isla Grande, fine sand, close to seagrass, from burrow of unknown host, bait suction (yabby) pump, 0.5-1 m depth, leg. A. Anker, 7.X.2006 [fcn 05-190].
Additional material examined, 2 breeding females (cl 4.7 mm, 5.6 mm), 2 post-breeding females (cl 4.4 mm, 5.1 mm), 4 subadults, 4 juveniles (cl not measured), USNM 1125421 About USNM , Tobago, Pirates Cove , sta. 2, bait suction (yabby) pump, 0-1 m depth, leg. R. Heard, 2.IV.1992 ; 3 breeding females (cl 4.5, 4.8, 5.0), 2 subadults, 1 juvenile (cl not measured), USNM 1125422 About USNM , Tobago, Sandy Bay , sta. 5, leg. R. Heard, 7.IV.1992 ; 1 breeding female [most eggs shed] (cl 5.9 mm, tl 14.7 mm), UO, Sta. MOBR-C-1546, EDIMAR-FLASA, Venezuela, Isla La Tortuga, Laguna Carenero, sand flat, associated with the ghost shrimp Neocallichirus grandimana , leg. J. Bolaños and C. Lira, 6. VI.2001; 1 breeding female (cl 4.5 mm, both P1 regenerating), USNM 1125423 About USNM , Colombia, Caribbean coast, Barú south of Cartagena, C. de los Vasquez, beach, bait suction (yabby) pump, leg. R. Lemaitre, 30.VII.1994 .
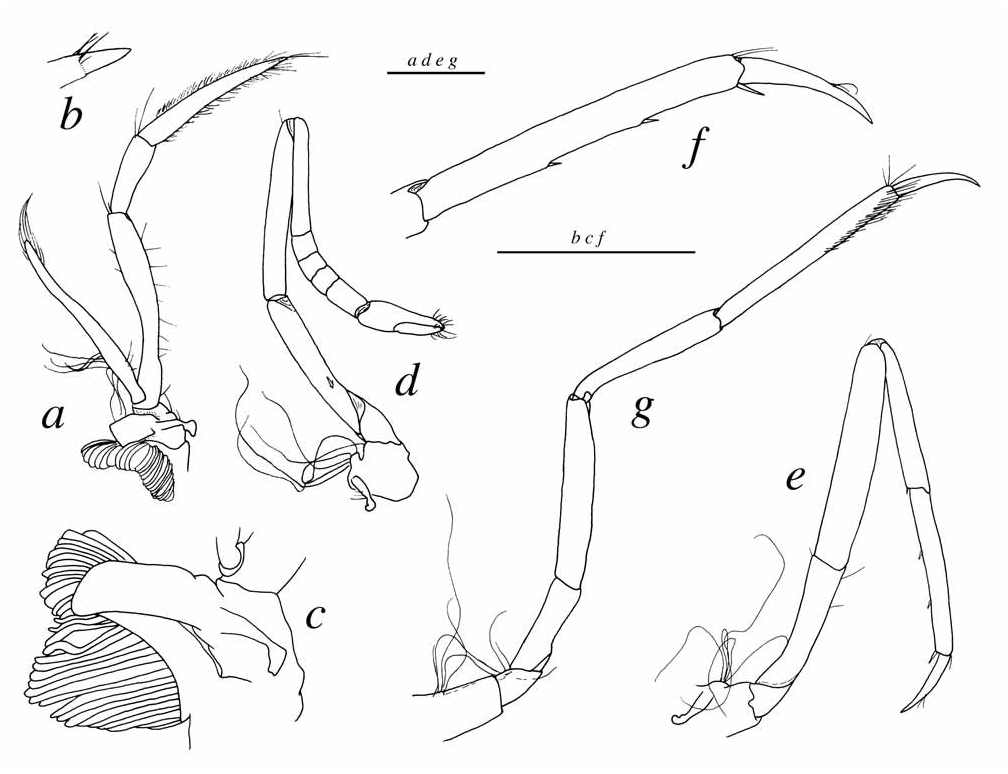
Description. Carapace minutely pitted, with sparse setae. Rostrum broadly triangular in dorsal view, about as long as broad at base, reaching distal margin of first segment of antennular peduncle, tip acute ( Fig. 7a View FIGURE 7 ); lateral margins shallowly concave; ventral margin with barely noticeable, blunt tooth subdistally ( Figs. 7b, d View FIGURE 7 ); rostral carina feebly developed, not reaching level of eyes posteriorly. Orbital teeth reduced to small blunt bumps ( Figs. 7a, c View FIGURE 7 ). Pterygostomial angle slightly protruding anteriorly, blunt ( Fig. 7b View FIGURE 7 ). Eyes concealed in dorsal and lateral views ( Figs. 7a–b View FIGURE 7 ); cornea distinctly reduced; anteromesial margin of eyestalk not protruding ( Fig. 7d View FIGURE 7 ).
Antennule with stylocerite reaching slightly beyond half-length of second peduncular, with acuminate tip; ventromesial carina of first segment with small sharp, anteriorly directed tooth ( Fig. 7e View FIGURE 7 ); second segment not elongate, as slightly longer than wide ( Fig. 7a View FIGURE 7 ); lateral flagellum bifurcating at third or fourth segment, secondary ramus well developed, with several groups of aesthetascs ( Fig. 7f View FIGURE 7 ). Antenna with basicerite bearing stout distoventral tooth ( Fig. 7b View FIGURE 7 ); scaphocerite broadly ovate-rectangular, with small acute distolateral tooth and broad blade, latter with slightly convex anterior margin and surpassed by distolateral tooth ( Fig. 7a View FIGURE 7 ); carpocerite stout, short, barely reaching half-length of scaphocerite.
Third maxilliped ( Fig. 8a View FIGURE 8 ) with rectangular, distally blunt lateral plate protruding towards and above hypertrophied arthrobranch ( Fig. 8c View FIGURE 8 ); ultimate segment tapering to corneous tip, without spines ( Fig. 8b View FIGURE 8 ).
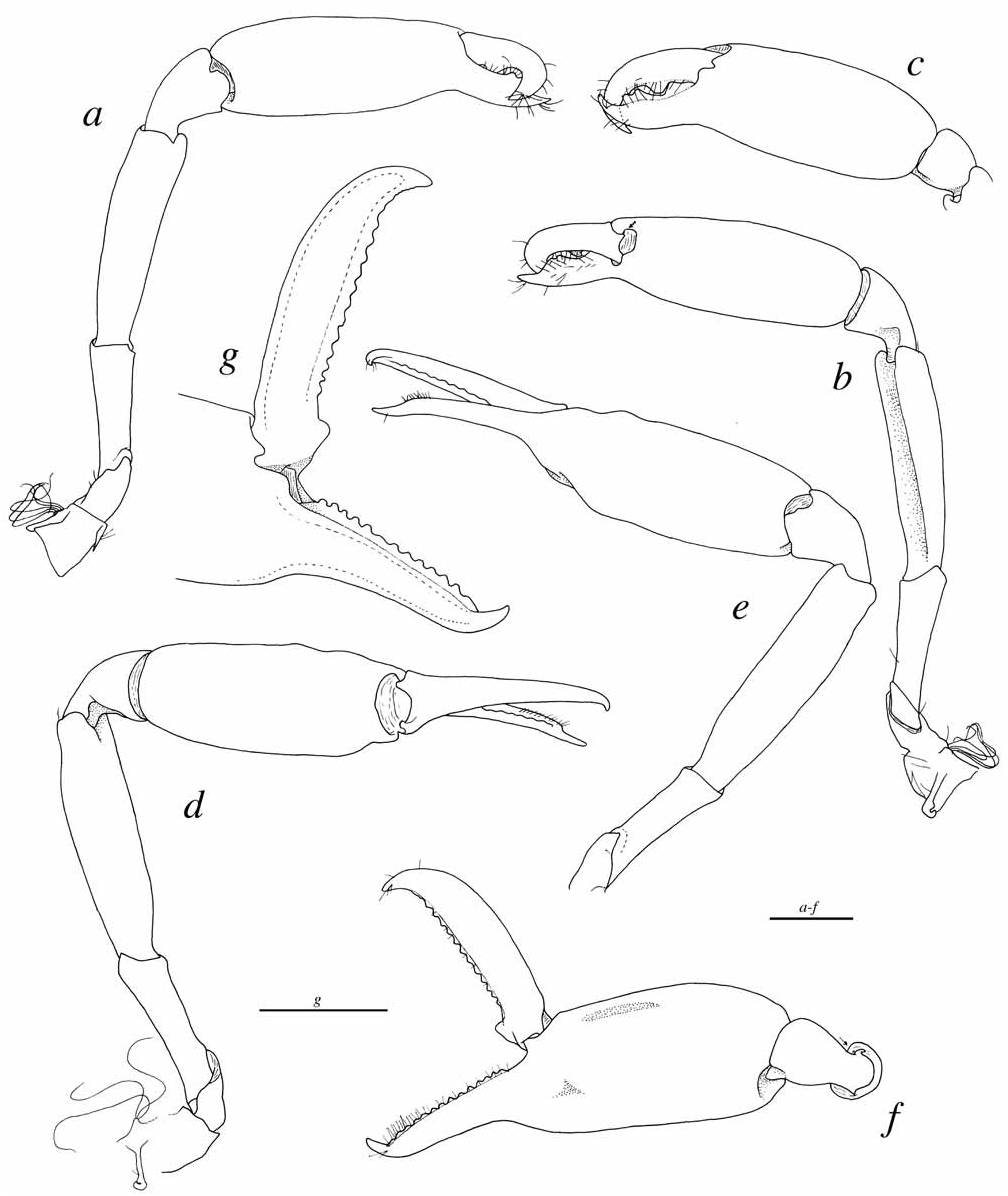
Chelipeds very asymmetrical in shape, but only slightly unequal in size ( Fig. 9 View FIGURE 9 ). Larger (= major) cheliped ( Figs. 9d–g View FIGURE 9 ) with ischium unarmed ( Figs. 9d–e View FIGURE 9 ); merus not inflated, depressed ventrally; carpus short, cupshaped; chela fairly slender, with fingers about 0.8 palm length; palm mostly smooth, except for distinctly marked groove (sinus) visible in dorsal or ventral view of chela, and adjacent, slightly protruding rounded bumps ( Figs. 9d–f View FIGURE 9 ); fingers slender, straight except for curved crossing tips, cutting edges serrated with at least 12 small rounded regular teeth; distal portion near finger tips unarmed ( Fig. 9g View FIGURE 9 ). Smaller (= minor) cheliped ( Figs. 9a–c View FIGURE 9 ) with ischium unarmed; merus only slightly less stout than in major cheliped, excavated ventrally; carpus slightly more elongate than in major cheliped, cup-shaped, with rounded lobes distally; minor chela stout, shorter than major chela, with fingers about half-length of palm, more strongly curved, especially at tips; cutting edge of dactylus armed with one strong tooth fitting into broad hiatus situated centrally on opposable cutting edge of pollex ( Fig. 9c View FIGURE 9 ), latter also bearing smaller proximal and subdistal teeth on cutting edge proximally and distally to central hiatus, respectively.
Second pereiopod ( Fig. 8d View FIGURE 8 ) relatively small, slender; ischium bearing one small spine on ventrolateral margin; merus longer than ischium; carpus five-segmented, first segment being approximately equal to sum of remaining four segments. Third pereiopod ( Fig. 8e View FIGURE 8 ) moderately slender; ischium apparently without spine(s) on ventrolateral margin; merus about five times as long as wide; carpus with short distoventral spinule or sera; propodus with two small ventral spinules and longer and stouter distal spinules; dactylus simple, conical, moderately slender, feebly curved, slightly more than 0.4 of propodus length ( Figs. 8e–f View FIGURE 8 ). Fourth pereiopod similar to third. Fifth pereiopod ( Fig. 8g View FIGURE 8 ) with very different proportions of ischium, merus, carpus and propodus; ischium and carpus unarmed; propodus unarmed, with well developed setal brush distally.
First to third pleura rounded; fourth and fifth pleura ending in rounded or blunt angle posteroventrally ( Fig. 7i View FIGURE 7 ); sixth pleurite without articulated plate, subacute posteroventrally; preanal plate triangular, rounded posteriorly ( Fig. 7j View FIGURE 7 ). Telson ( Fig. 7l View FIGURE 7 ) about twice as long as proximal width, slightly tapering posteriorly, with two pairs of small dorsal spines situated first at mid-length, second at 0.75 of telson length; posterior margin almost straight, with central portion slightly concave, but without distinct abrupt notch, with two pairs of thick plumose setae in central portion, fringed by two pairs of stout spines, lateral being somewhat shorter than mesial ( Fig. 7m View FIGURE 7 ).
Second pleopod with appendix masculina shorter than appendix interna, furnished with stout setae apically ( Fig. 7h View FIGURE 7 ). Uropod ( Fig. 7k View FIGURE 7 ) with relatively broad exopod and endopod; diaeresis sinuous; distolateral tooth and distolateral spine both fairly stout.
Gill/exopod formula typical for genus (see under first species).
Size. Holotype cl 5.6 mm, tl 15.0 mm; cl of adult specimens ranges from 4.4 to 5.9 mm.
Colour in life. Semitransparent, whitish; gonads and embryos yellow ( Fig. 14c View FIGURE 14 ).
Etymology. Named for Sammy De Grave (Oxford University Museum of Natural History), a good friend and colleague, and a well-known taxonomist of caridean shrimps.
Type locality. Isla Grande, Panama.
Distribution. Presently known from the Caribbean coast of Panama ( Isla Grande), Colombia (Cartagena area), Venezuela ( Isla Tortuga), and Tobago.
Ecology. Near-shore silt-sand flats, near seagrass, at depths of 0.5 m or less, associated with burrows. The host of the Venezuelan specimen was identified as Neocallichirus grandimana ( Gibbes, 1850) , a large ghost shrimp from the family Callianassidae . The original field notes by R. Heard indicate that at least some Tobago specimens were commensal with Callianassa branneri Rathbun, 1900 , a junior synonym of N. grandimana (see Manning 1993). The holotype specimen from Panama was not collected together with its host, but numerous specimens of Neocallichirus cf. grandimana were collected at the same locality, some only a few meters away from the collection site of S. degravei sp. nov. (A. Anker, pers. obs.; see also Anker 2008). All this evidence strongly suggests that Neocallichirus grandimana may be the host of S. degravei sp. nov. throughout its distribution range.
Variation. In the majority of the Tobago specimens, the minute blunt tooth on the ventral margin of the rostrum that is present in the holotype ( Fig. 7d View FIGURE 7 ) is not distinguishable. Similarly, in many specimens from Tobago, one or both orbital teeth are reduced to very small bumps ( Fig. 7n View FIGURE 7 ). In many specimens, the tip of the third maxilliped bears a stout spine-like subterminal seta.
Remarks. Salmoneus degravei sp. nov. is closely related to S. caboverdensis Dworschak, Anker & Abed- Navandi, 2000 from Cape Verde Islands, and S. erasimorum Dworschak, Anker & Abed-Navandi, 2000 from Croatia, but differs from both of them by the much less developed orbital teeth and the absence of spines on the ischia of both major and minor chelipeds (for major/minor cheliped assignment see below); specifically from S. erasimorum by the continuous, saw-like teeth on the cutting edges of the major chela (vs. distinctly spaced teeth in S. erasimorum ); and from S. caboverdensis by the posterior margin of the telson being straight (vs. having a small median notch in S. caboverdensis , and the carpus of both chelipeds being unarmed ventrally (vs. with a strong, sharp ventral tooth in S. caboverdensis ) (cf. Figs. 7–9 View FIGURE 7 View FIGURE 8 View FIGURE 9 and Dworschak et al. 2000: figs. 27–56). The new species is very different from the other two eastern Atlantic species of Salmoneus with subequal chelipeds, viz. S. jarli ( Holthuis, 1951) from West Africa, and S. sketi Fransen, 1991 from submarine caves off Croatia (see Holthuis 1951; Fransen 1991). On the other hand, S. degravei sp. nov. shows more similarity to the recently described S. brucei Komai, 2009 from the western Pacific ( Komai 2009), which shows no trace of orbital teeth. However, S. brucei differs from S. degravei sp. nov. by the presence of setal rows on the ventral margin of the pollex and dorsal margin of the dactylus of the major chela, and the unusually stout tooth on the lateral portion of the uropodal diaeresis (see Komai 2009).
Salmoneus degravei sp. nov. superficially resembles Deioneus sandizelli Dworschak, Anker and Abed- Navandi, 2000 from Cape Verde in both the general appearance and the infaunal life style ( Dworschak et al. 2000), but can be separated from it by the absence of a distinct articulated flap on the sixth pleurite and the presence of a well-developed appendix masculina on the second pleopod (including in breeding specimens, Fig. 7g View FIGURE 7 ), two features used by Dworschak et al. (2000) to separate Deioneus from Salmoneus . The new species also differs from D. sandizelli by the different tooth armature on the chelipeds and the presence of a small spine on the ischium of the second pereiopod, which is lacking in D. sandizelli ( Figs. 8d View FIGURE 8 , 9 View FIGURE 9 and Dworschak et al. 2000: figs. 15-19).
Anker & Marin (2006) grouped four eastern Atlantic species in the Salmoneus jarli species group: S. jarli , S. caboverdensis , S. erasimorum , and S. sketi . Since S. degravei sp. nov. shares many characters with S. caboverdensis and S. erasimorum , the new species would be “automatically” included in the S. jarli species group. This group was mainly characterised by the relative development of the chelipeds, with one cheliped being stout but short, and the other slender but long. However, one Indo-West Pacific species, S. seticheles Anker, 2003 , is known to have polymorphic chelipeds: some individuals have typical, non-enlarged minor cheliped, whereas others have an enlarged minor cheliped with a stout chela and fingers armed with strong teeth, termed “subminor” cheliped ( Anker 2003b). In addition, in S. jarli , the finger cutting edges of both chelae are without serrations or other type of teeth ( Holthuis 1951), a unique feature within the genus Salmoneus , suggesting that S. jarli may not be necessarily closely related to the other four species of the S. jarli group. On the other hand, Deioneus sandizelli shares a number of characters, including the relative development and the general shape of the chelipeds, with S. caboverdensis , S. erasimorum , S. degravei sp. nov., and to a lesser extent, also with S. sketi . Therefore, the S. jarli species group in its present definition may be not be monophyletic and there is also a possibility that Deioneus may be embedded within Salmoneus , in a clade comprising S. caboverdensis , S. erasimorum and S. degravei sp. nov.
The assignment of major/minor chelipeds in these particular species of Salmoneus and in Deioneus can be somewhat problematical. In the above description of S. degravei sp. nov., the smaller and stouter cheliped is called “minor”, whilst the larger and more slender cheliped is called “major”. To enable direct comparisons and to avoid future confusions, the major/minor cheliped should be assigned as in the description of S. degravei sp. nov., based on the cheliped length, independently from its slenderness or stoutness. Therefore, the assignment of the major/minor chelipeds should be as following:
S. jarli : “longer and more slender”, “smaller first pereiopod” ( Holthuis 1951: fig. 20m) = major cheliped; “short and robust”, “larger first pereiopod” (ibid.: fig. 20k, l) = minor cheliped.
S. sketi : “left first pereiopod” ( Fransen 1991: figs. 15–16) = major cheliped; “right first pereiopod” (ibid.: fig. 14) = minor cheliped.
S. caboverdensis : “minor cheliped” ( Dworschak et al. 2000: fig. 29) = major cheliped; “major cheliped” (ibid.: figs. 30,–31) = minor cheliped.
S. erasimorum : “minor cheliped” ( Dworschak et al. 2000: figs. 46–47) = major cheliped; “major cheliped” (ibid.: figs. 44–45) = minor cheliped.
S. degravei sp. nov.: major cheliped: Figs. 9d–g View FIGURE 9 ; minor cheliped: Figs. 9a–c. View FIGURE 9
S. brucei : major cheliped ( Komai 2009: fig. 3); minor cheliped (ibid.: fig. 4).
S. seticheles : major cheliped ( Anker 2003b: fig. 3A–C, 4A); typical minor cheliped (ibid., figs. 2I–J); subminor cheliped (ibid., fig. 4B).
D. sandizelli : “minor cheliped” ( Dworschak et al. 2000: figs. 15–16) = major cheliped; “major cheliped” (ibid.: figs. 17–18) = minor cheliped.
| R |
Departamento de Geologia, Universidad de Chile |
| UO |
University of Oklahoma |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.