Macrosiphum edrossi Essig, 1953
|
publication ID |
https://doi.org/10.5281/zenodo.11066923 |
|
DOI |
https://doi.org/10.5281/zenodo.11092795 |
|
persistent identifier |
https://treatment.plazi.org/id/244487FD-4017-FFCA-FE58-FCFCAA0AFA5E |
|
treatment provided by |
Felipe |
|
scientific name |
Macrosiphum edrossi Essig, 1953 |
| status |
|
Macrosiphum edrossi Essig, 1953 View in CoL
1953 Macrosiphum edrossi Essig. Proceedings of the Californian Academia of Sciences, ser. 4, 28 (3):118.
MATERIAL EXAMINED.— PERU, Ayacucho department, Ayacucho, near Pampas River , 8 March 1951, on “nettle?”, A.E. Michelbacher leg., E.O. Essig det., 13 viviparous apterous females and 6 alate viviparous females (4 apt. and 3 al. in CAS collection and 9 apt. and 3 al. in NHM collection), paratypes . PERU, Cusco department, Pisac (13º26ʹS 71º50ʹW and 2,980 m a.s.l. aprox.), 25 May 2010, on Baccharis latifolia, J. Ortego leg., J.M. Nieto Nafría & J. Ortego det., 4 apterous viviparous females, Universidad de León collection .
REDESCRIPTION.— From above mentioned specimens and original description by Essig (1953).
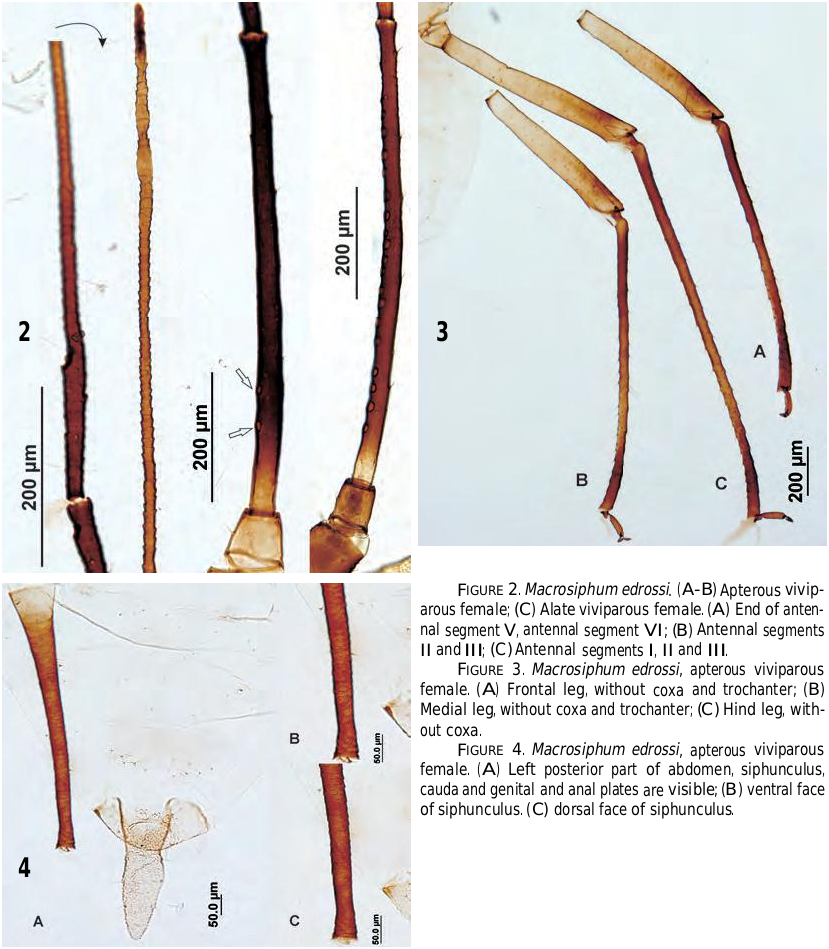
Apterous viviparous females (Figs. 1, 2A, 2B, 3, 4).— Color when alive pale green with brown antennae, legs and siphunculi. When mounted very light yellow, with head including clypeous and mandibular and maxillary lames, rostrum, legs, siphunculi, anal plate and cauda more or less pigmented (Fig. 1A). Body length, 2.325 –3.275 mm (3.38–4.65 times siphunculus) including cauda and 2.025 –2.950 mm (2.97–4.05 times siphunculus) without cauda. Head brownish yellow, and smooth, with 2+2+4 dorsal setae in addition to the other four placed on the edge of the frons (Fig. 1B); they are 22–35 µm and 0.5–0.9 times subarticular diameter of the antennal segment III [from here D], fine, pointed and very pale. Ventral setae similar in shape and pigmentation to dorsal ones and somewhat longer than them. Frontolateral tubercles tall, divergent, apically rounded and marking a frontal sinus, and frontomedial tubercle lower than those (Fig. 1B). Antennae 3.398 –4.480 mm and 1.25–1.54 times body length with cauda (1.39–1.75 times without cauda). Antennal segments I and II smooth and colored like head (Fig. 1A), with setae similar in shape, length and lack of pigmentation to those of head. Antennal segment III, 0.73–0.92 mm, near smooth, very dark brown except a small basal portion (8.2–13.8% of its total length) that is pale like head ( Fig. 2B View FIGURE ); 2–9 secondary sensoria, small, more or less circular and aligned on the 2/3 of the dark part of segment at most. Segment IV, 0.53–0.81 mm, softly imbricated and dark brown; segment V, 0.43–0.65 mm, imbricated and brown to dark brown; both two without secondary length), progressively imbricated from its near smooth widened basal portion to apex, with 2–4 lines of small cells on distal 3.8–6.3% of their total length. Genital plate very pale and with setae as usual. Cauda (Fig. 4) lanceolate, pigmented than head and anal plate, 0.30–0.43 mm, 0.5–0.6 times siphunculus, 1.7–1.9 times its basal width, and with 6–8 long, curved, delicate and pointed setae.
Alate viviparous females ( Fig. 2C View FIGURE ).— When alive “yellowish or greenish […] with head, thorax and all appendages brown to black” from Essig (1953), appendages must be understood: antennae, legs and siphunculi. Prepared specimens very similar to apterous viviparous females, excluding the thoracic organization (sclerites and wings) and pigmentation (yellowish brown). Other appreciable differences as rapport of apterae are as follows; minimum and maximum limits of not mentioned metric and meristic characteristics are included within the range for each feature in apterae. Antennal segments I and II more pigmented. Antennal segment III, 0.72–0.83 mm; pale proximal portion 7.5–11.3% of total length, carrying 14–19 secondary sensoria extended on two third of the segment length ( Fig. 2C View FIGURE ). Processus terminalis of antennal segment VI, 1.18–1.33 mm, 6.0–7.1 times base and 1.4–1.7 times segment III; ultimate rostral segment, 0.15–0.17 mm. Femora more pigmented, in extension and intensity. Dorsal setae of middle third of hind tibia, 0.6–0.9 times the width of segment at point of insertion. Marginal sclerites on abdominal segments 2–4, conspicuous, spinuled, sometimes pigmented and carrying 4–5 setae 13–28 µm and 0.3–0.7 times D. Setae on abdominal segment 8, 27–40 µm and 0.7–1.7 times D. Siphunculus, 4.28–4.92 times and 3.93–4.50 times included in body length with cauda and without cauda respectively; reticulation provided of 4–5 lines and extended on 6.5–7.8% of total length of siphunculus. Cauda, 2.0–2.3 times its basal width.
BIOLOGY, PLANT HOST.— Two plant species have been mentioned as host plants for Macrosiphun edrosii: Urtica sp. (?) by Essig (1953) from Michelbacher’s collection data, and Baccharis latifolia (Ruiz and Pav.) Pers. ( Asteraceae, Astereae ) in this paper from Ortego’s collection data. Both plants have different aspects and in addition B. latifolia cannot be confused with a nettle because it lacks the characteristic stinging trichomes. It could be that the specimens collected by Michelhacher were vagrants, although there are many specimens to establish it with certainty, and it could also be that they were collected when beating plants of nettle among which there were twigs of B. latifolia that could have gone unnoted; we note that Michebacher collected the type specimens of Delfinoia peruviana ( Essig, 1953) when beating onto a canvas in the same locality and date. Nevertheless, it is also possible that both plant taxa are hosting M. edrossi , because oligophagy or polyphagy are possible in aphids.
The life cycle of the species is unknown, but it would be holocyclic (with sexual generation and winter eggs) to be able to withstand the low winter temperatures of the areas where it has been found.
GEOGRAPHICAL DISTRIBUTION.— Macrosiphum edrossi is currently only known in Ayacucho and Pisac ( Peru), which are separated one from another about 280 km in a straight line, but it is also very likely that its distribution is much more extensive, because Baccharis latifolia is known in Argentinean North-West, Bolivia, Peru, Ecuador, Colombia and Venezuela.
TAXONOMIC DISCUSSION.— Although most of the characters of viviparous females of Macrosiphum edrossi suggest it should be placed in the genus Macrosiphum , it must be noted that the small surface occupied by the siphuncular apical reticulation is a very peculiar character that generates doubts about this taxonomic assignment. An analysis of nucleic sequences would certainly help clarify its taxonomic status, but it will be necessary to wait to have material fixed in suitable conditions to be able to obtain them. Doubts could be solved if other presumably South American native species sufficiently similar to it were to be discovered.
| CAS |
California Academy of Sciences |
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |