Chrysocharis laomedon ( Walker, 1839 )
|
publication ID |
https://doi.org/ 10.1080/00222933.2012.654517 |
|
persistent identifier |
https://treatment.plazi.org/id/211587B2-3B0D-FFD5-FE1F-FD4CC0E5FA7E |
|
treatment provided by |
Felipe |
|
scientific name |
Chrysocharis laomedon ( Walker, 1839 ) |
| status |
|
Chrysocharis laomedon ( Walker, 1839) View in CoL
Chrysocharis laomedon (Walker) View in CoL : Graham 1963, p. 210.
Type material
Lectotype ♀, designated by Hansson (1985) (Natural History Museum, London; not examined).
Material examined
34 ♀♀, 27 ♂♂, C. laomedon : Ul’yanovsk, Park Vinnovskay Roshcha , 54 ◦ 16 ′ N; 48 ◦ 20 ′ E, 21 June–22 August 2009 (A. Mishchenko) GoogleMaps .
Comparative notes
Malar space narrow, mid lobe of mesoscutum and scutellum purple, legs white or very pale yellow, scutellum distinctly longer than wide, gaster about as long as thorax + propodeum ( Hansson 1985).
Distribution
Czech Republic, Denmark, Finland, France, Germany, Great Britain, Hungary, Japan, Moldova, Netherlands, Poland, Russia, Sweden, Switzerland, Yugoslavia ( Noyes 2010).
Biology
Larval-pupal solitary endoparasitoid.
Remarks
The investigations have shown that P. issikii has either two or three generations in Ulyanovsk province and that 100% of trees of Tilia cordata were attacked by P. issikii . The overwintering moths (females and males) begin to fly at the end of May. The fertilized females lay their eggs on the underside of the leaves, and often different females will oviposit on the same leaf, resulting in the leaves of both species of Tilia having several mines of P. issikii , so that the overall extent of the mines is large. The first generation of moths can mine the upper side of the leaves, but this happens rarely, and only in July on T. cordata . Moths of the second generation occur from the last 10 days of July to the first 10 days of August. The leaf miners of the third generation that develop on T. cordata fly from the second 10 days of August to the first 10 days of September. The first mines of P. issikii on T. cordata appear in June and the last in September.
The larval mines begin to appear 5–7 days after oviposition, the larvae feeding on the mesophyll of the leaves for 7–10 days and forming characteristic, blotchshaped mines. Pupation takes place inside the mine and the adult moth emerges after 8–10 days, the life cycle being repeated two or three times per year.
The development time of the reared parasitoids is not longer than, or is about as long as, that of P. issikii . The development time of M. frontalis is 1.3×, and that of C. laomedon 1.6×, shorter than that of P. issikii .
The female of M. frontalis attacks larvae of the first to fifth instars, or the pupa, of P. issikii .
Description of preimaginal stages of Minotetrastichus frontalis
Egg
The egg of M. frontalis is laid beside the third instar larva of P. issikii (distance between host larva and egg 0.2 mm) in its mine on Tilia cordata ( Figure 1 View Figure 1 ) (four observations). The egg is 0.3 mm long, with both ends rounded, but one is a little broader than the other. The egg is white and shiny, without sculpture.
1st instar larva
Morphology
The larva of the first instar (1 mm) ( Figure 2 View Figure 2 ) has 13 distinct segments. The II–IV thoracic and VI, VIII, X and XII abdominal and XIII anal segments have two short protuberances arising from both sides, with long setae, the first and last seta are the shortest. The length of the setae on these segments is about equal to the breadth of larva. Abdominal segments V, VII, IX, and XI are without setose protuberances. The larva uses these setose protuberances for moving.
Behaviour
The larva is active in the mine of the host and jumps onto the surface of the host, and very slowly starts to feed. The larva does not have a specific place for localization on the host’s body, and is also capable of feeding on larvae or pupae of P. issikii (115 observations).
2nd instar larva
Morphology
The larva of the second instar (2 mm) ( Figure 3 View Figure 3 ) is 2× as long as the previous stage and has lost the long seta on the protuberances, but the latter are still prominent (84 observations).
Behaviour
In this stage the larva is actively feeding; the gut has visible peristaltic movements that begin from the thoracic segments to the caudal abdominal segments. At the end of this stage the protuberances begin to be distinctly visible. The host is in its 4th or 5th instar when the parasitoid reaches its 2nd instar ( Figure 4 View Figure 4 ). Gregarious parasitism is distinctly visible in this larval stage ( Figure 5 View Figure 5 ) on prepupae of the host, and we observed a minimum of two, and a maximum of five, larvae on one host larva. The larvae never exhibited siblicide behaviour, and in fact the food was apparently distributed among the larvae. Larvae of one brood of M. frontalis had very close contact because of the small space on the host larva and in our investigation we have never documented antagonistic behaviour towards each other. We never observed more than five larvae of M. frontalis on the host; probably this number of larvae on this host is optimal for the gregarious strategy.
In this stage, larvae of M. frontalis can be hyperparasitoids of braconids ( Apanteles sp. ) (see Figure 6 View Figure 6 ). M. frontalis completes its development successfully more often on Phyllonorycter larvae, and only in 27% cases it is developed as a hyperparasitoid of Apanteles sp. (Yefremova and Mishchenko 2008). Such hyperparasitism is facultative (40 observations). The moth host larva is not large enough, and the food is insufficient for development of all parasitoids in parasitoid complex, in that the average number is from four to eight parasitoids (Yefremova and Mishchenko 2008).
Behaviour
Larvae of this stage are feeding so intensively that they might even eat sibling larvae or prepupae ( Figure 7 View Figure 7 ). One cannot call this event siblicide, because these larvae are from different females of M. frontalis . Sometimes the female of M. frontalis lays an egg in a larva of P. issikii that is already parasitized – this is superparasitism.
3rd instar larva
Morphology
The third instar larva (4 mm) ( Figure 8 View Figure 8 ) is 2× as long as larvae of previous stages, with distinct protuberances on the same segments (II–IV thoracic and VI, VIII, X and XII abdominal and XIII anal segments) as in the second instar. The protuberances are located on both sides of each thoracic segment and on VI, VIII, X and XII abdominal segments; V, VII, IX, XI without protuberances. Each small protuberance has trichoid setae (Figure 9) that may have a sensory function. The colour of the larva at this stage varies from white, pale green to dark yellow and brown.
Behaviour
The larvae feed on haemolymph, and are capable of feeding on the soft cuticle of the host (156 observations).
4th instar larva / prepupa
Morphology
The larva of the fourth instar (5 mm) ( Figure 10 View Figure 10 ) has lost its mobility and has eight pairs of spiracles clearly visible on abdominal segments I–VI (101 observations).
Figure 9. Protuberances of larva of the fourth instar of M. frontalis (A) and thrichoid seta (B, magnification 320×) (Photo: A.V. Mishchenko, 5 September 2009).
At the end of this stage we could observe the active process of histogenesis ( Figure 11 View Figure 11 ) and a “pillar” of faecal pellets ( Figure 12 View Figure 12 ). Segmentation of the body is not visible, and after excretion the larva prepares to pupate. The prepupa does not move and lacks protuberances and filaments. Thus, M. frontalis has four larval instars and three moults. The last instar is 5× as long as the first instar (116 observations).
No larval stage has a specific localization on the surface of the host larva or pupa. The size of host and its cuticle was never a barrier for attack by M. frontalis .
Behaviour
The parasitoid larva always shifts its feeding position (probably after intensive feeding on the haemolymph at the initial site).
The immature larva of M. frontalis is 2–3 times as long as the host larva. Facultative hyperparsitism is common for the 2nd–4th instar larvae (24 observations).
When the host larva was attacked by eulophids of different species belonging to the genera Chrysocharis , Pnigalio and Sympiesis (multiparasitism), we observed antagonistic behaviour among them, with the death of the older larvae instead of the youngest. However, it is very important to mention that larvae of M. frontalis might appear together (i.e. they can be gregarious) and could put pressure on larvae of other parasitoids and kill should they be present; however, the latter were never used as food.
Behaviour
Antagonistic behaviour against larvae of the same species was observed; larvae of the first and second instar will attack larvae of the fifth instar, the prepupa and the pupa.
Pupa
Morphology
The pupa (5 mm) ( Figure 13 View Figure 13 ) is pale brown and attached by a filament to the leaf. The duration of the pupal stage is on average 10 days.
Behaviour
Emergence of the adult occurs as usual in the early morning (based on several observations) ( Figure 14 View Figure 14 ) and they chew through the epidermis of the leaf and fly away. The total development time of M. frontalis is 11.2 days (203 observations) (SE = ±0.41).
Description of preimaginal stages of Chrysocharis laomedon
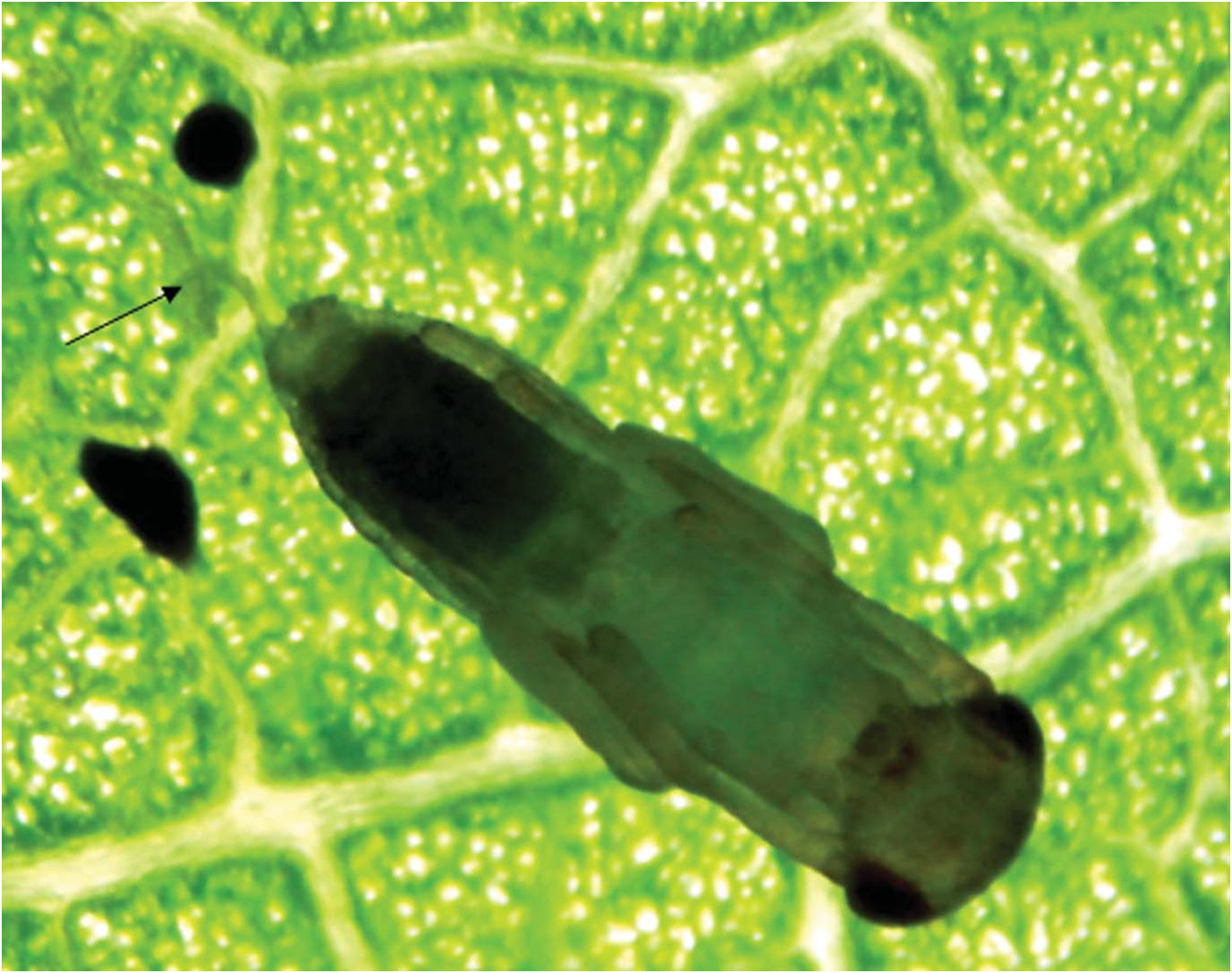
The female of C. laomedon always attacks the immature larvae of the fourth instar of P. issikii . Before oviposition, the female paralyses the larva of P. issikii , and we could distinctly see melanized marks probably caused by the parasitoid’s ovipositor on the 11th body segment ( Figure 15 View Figure 15 ). The female of C. laomedon lays eggs inside the cuticle of the host’s larva.
Egg
Morphology
The egg is very small, 0.1–0.3 mm, and continues to develop over a period of three days under the cuticle of the host larva; the newly hatched larva goes to the outside of the cuticle. After paralysis, the host stops developing (25 observations).
1st instar larva
Morphology
The first instar larva is 2 mm long ( Figure 16 View Figure 16 ). The segments are not clearly discernible, but the head has easily recognizable characters. It continues development on
the surface of the host larva. We have observed the parasitoid larvae to be located on segments 3–5 of the larva of P. issikii .
Behaviour
The larva feeds on the haemolymph of the host ( P. issikii ) and moults to the second instar on the surface of its body (53 observations).
2nd instar larva
Morphology
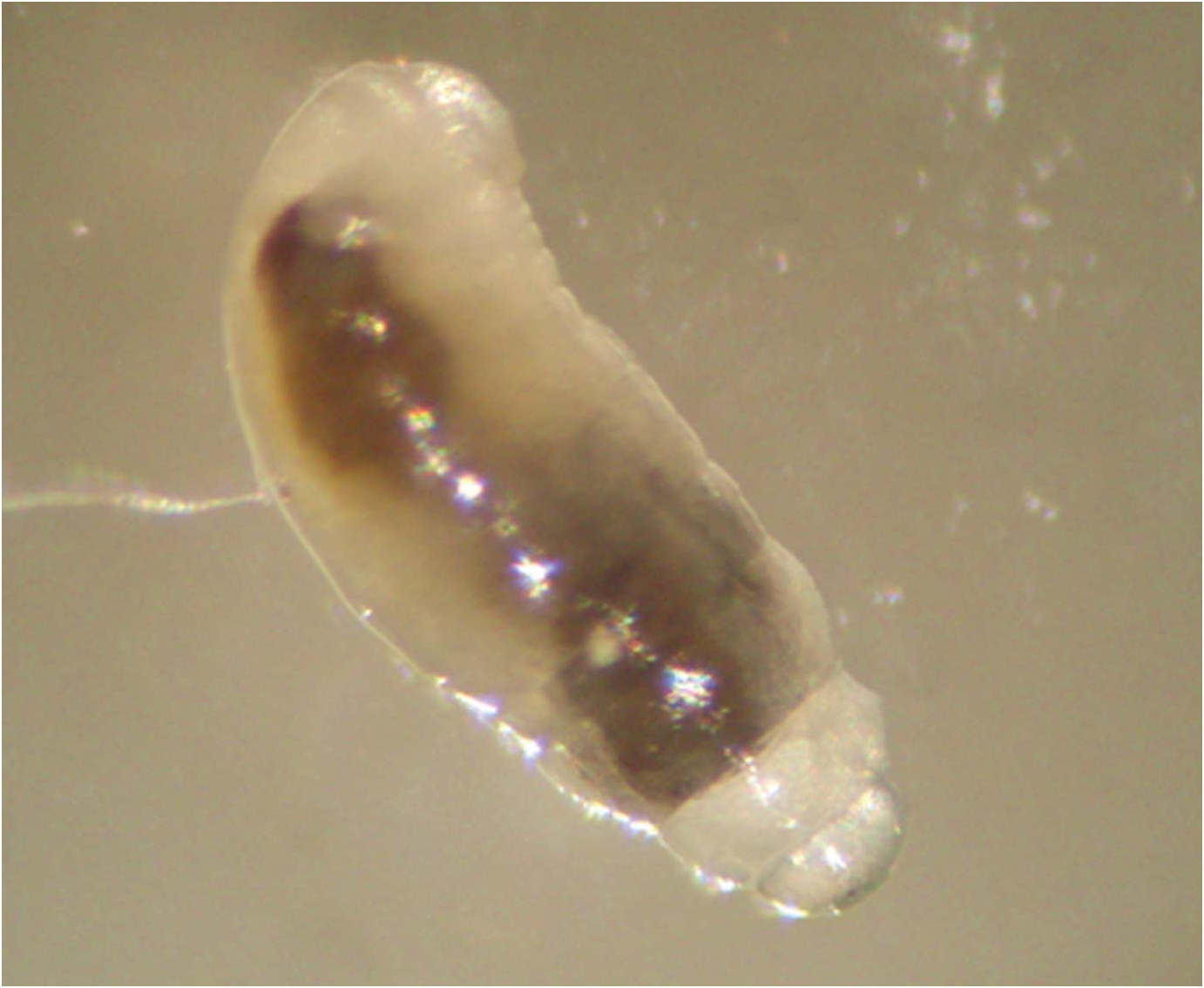
The larva of the second instar is 4 mm in length ( Figure 17 View Figure 17 ) and does not have any protuberances; its body segmentation is weaker than that of the first instar.
Behaviour
It also feeds on the haemolymph of the host but not at any specific place. The second instar larva is more active than that of the first instar. It moults into the final (third) instar outside the host larva (48 observations).
3rd instar larva
Morphology
The third instar larva (6 mm) ( Figure 18 View Figure 18 ) stops feeding and gradually loses its mobility. In this stage the respiratory system (tracheal system) and a seven external openings called spiracles on I–VII segments) is clearly visible. The larva has lost the segmentation of the body. The size of the fully grown larva is equal to that of the host larva.
Behaviour
When fully fed, the parasitoid larva leaves the host’s remains and normally pupates completely free of them in 2–3 days (65 observations).
4th instar larva / prepupa
Morphology
The prepupa ( Figure 19 View Figure 19 ) excretes a “pillar” of faecal pellets in the mine of P. issikii , and begins to form a pupal cuticle. The pupa ( Figure 20 View Figure 20 ) (6 mm) (97 observations) has a hard, opaque chitinized surface through which the parasitoid is not visible. The parasitoid pupa may be found beside the remnants of the host.
Total development is 20.5 days (118 observations) (SE = ±0.48).
Behaviour
Emergence of the adults ( Figure 21 View Figure 21 ) is early in the morning; the adult chews through the epidermis of the leaf and flies away.
We have never observed the female of C. laomedon laying eggs in host larvae that were previously parasitized by a female of the same species. C. laomedon has three larval instars and two moults. The size of the last instar is three times as long as the first instar.
Conclusion
Both parasitoids M. frontalis and C. laomedon can be found attacking the same host, P. issikii , and their development takes place inside a leaf mine on Tilia . The two species have different developmental characteristics ( Table 2).
In spite of these characteristics, both parasitoids experience the same environment inside the mine, with the same temperature and humidity, and both species might have the same adaptation to parasitism in the leaf mine. Because of the high humidity inside the mine, and a fairly constant temperature under laboratory conditions, it is suggested that C. laomedon has changed from the typical behaviour of endoparasitoids and, as a consequence, the development of its larva and pupa is external, i.e. on the surface of the host larva, P. issikii ; moreover, the larva is not exposed to the risk of dehydration. Such a situation enabled us to study the structure of the endoparasitoid larva without dissecting the host larva, which is difficult. Developing inside the mine of P. issikii , ectoparasitoids and endoparasitoids have similar behaviours but a different strategy of parasitism; the first allows the host to develop, the second prevents it from developing. The solitary endoparasitism that was discovered during our investigation is a different strategy than gregarious idiobiont ectoparasitism because the first does not allow the host larva to live as long; therefore C. laomedon might have changed to a strategy that allows it to develop inside the host larva or to develop outside. This is in comparison to ectoparasitism that is evolutionarily another strategy with morphological adaptations (in larval stages) and a short period of development. The present study highlights the important role of morphological investigation of preimaginal stages for understanding the adaptation to parasitism. If M. frontalis is present as a hyperparasitoid of Apanteles , it does not suppress the moth but indirectly decreases the influence of Apanteles by reducing their numbers in the complex. Lastly, it is evident that differing parasitoid strategies potentially have an important influence on the use of parasitoids in biological control.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chrysocharis laomedon ( Walker, 1839 )
| Yefremova, Z. & Mishchenko, A. 2012 |
Chrysocharis laomedon (Walker)
| Graham MWR de V. 1963: 210 |