Epeolus boliviensis Friese, 1908
|
publication ID |
https://doi.org/10.5852/ejt.2019.563 |
|
publication LSID |
lsid:zoobank.org:pub:6F6E082D-0675-49C1-A603-F7BABB546C46 |
|
DOI |
https://doi.org/10.5281/zenodo.3477519 |
|
persistent identifier |
https://treatment.plazi.org/id/201E87AD-FF95-FFDB-1852-FB2D40808D2B |
|
treatment provided by |
Plazi |
|
scientific name |
Epeolus boliviensis Friese, 1908 |
| status |
|
Epeolus boliviensis Friese, 1908 View in CoL
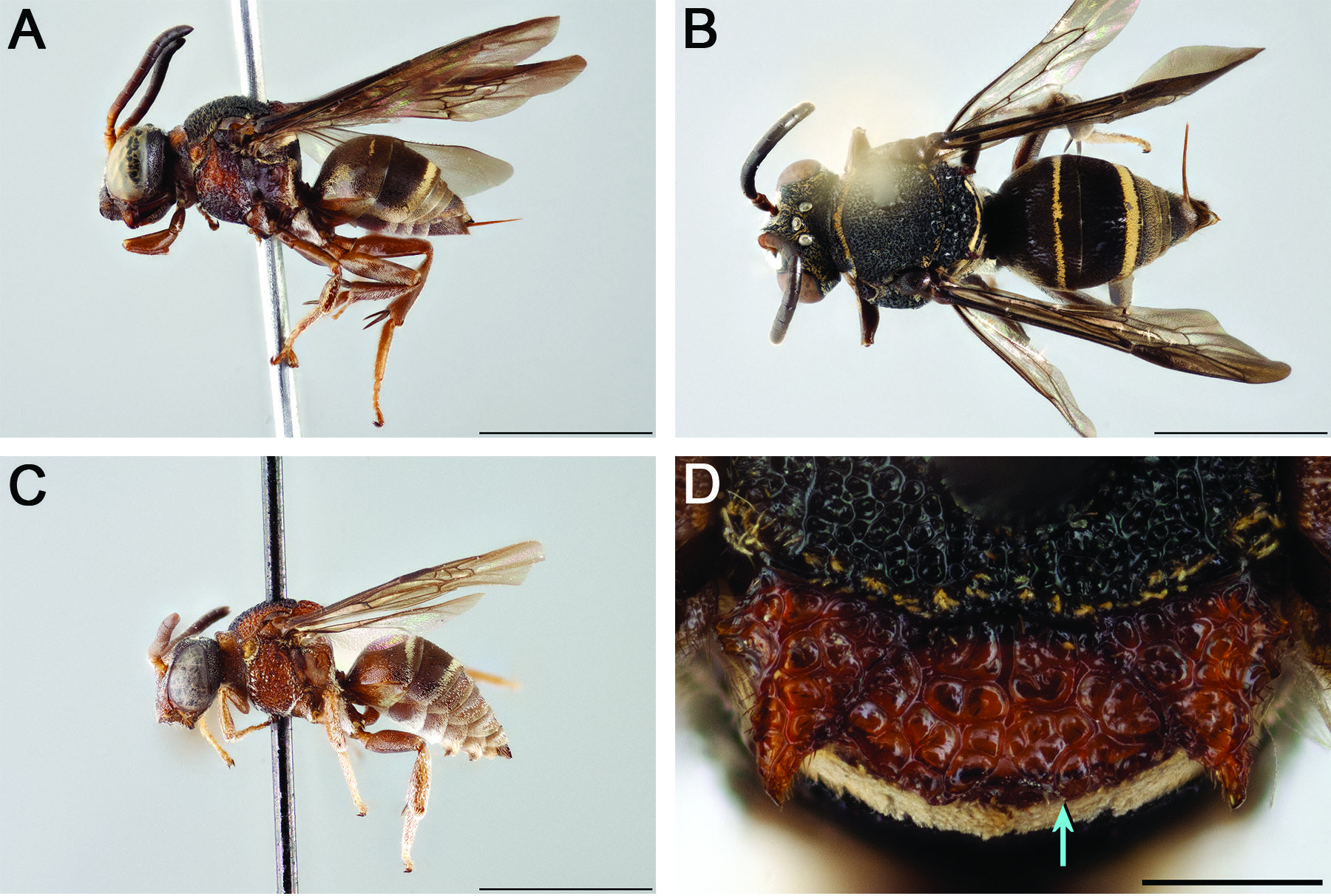
Figs 1A View Fig , 2A View Fig , 6 View Fig , 7A View Fig
Epeolus boliviensis Friese, 1908: 88 View in CoL ( ♂).
Trophocleptria schraderi Michener, 1954: 127 ( ♀), syn. nov.
Proposed common name
Bolivian epeolus.
Diagnosis
Together with the morphological features that are diagnostic for the ‘ Trophocleptria group’, the following in combination can be used to tell E. boliviensis apart from all other Epeolus : the axillae are crenulate along the lateral margin, each with a large tooth near the base ( Fig. 6D View Fig ); the mesoscutellum has a pair of posteriorly directed teeth ( Fig. 6D View Fig ); the fore wings are deeply infuscate apically ( Fig. 6 View Fig A–B); T1 has only a bright to pale yellow subapical fascia, which is usually narrower than the T2 apical fascia ( Fig. 6B View Fig ); and the remaining terga lack fasciae, although the apical impressed areas occasionally have sparse, off-white hairs ( Fig. 6 View Fig A–C). In overall appearance, this species is more nomadiform than epeoliform ( sensu Michener 2007). Epeolus boliviensis most closely resembles E. fulvopilosus Cameron, 1902 and E. nomadiformis sp. nov., but in E. fulvopilosus T3 and T4 are distinctly fasciate and in both E. fulvopilosus and E. nomadiformis sp. nov. T1 has a broad, medially narrowed or interrupted bright to pale yellow basal fascia, which in E. boliviensis is lacking entirely.
Material examined
Primary type material
BOLIVIA • ♂, E. boliviensis holotype; La Paz Department, Mapiri; 1900; ZMB .
COSTA RICA • ♀, Tro. schraderi holotype; Limón?, Los Diamantes ; 26 May 1948; F. Schrader leg.; KUNHM SEMC1461052 .
DNA barcoded material with BIN-compliant sequences
Unavailable.
Non-barcoded material
ARGENTINA • 1 ♂; Tucumán Province, 12 km N of El Cadillal ; 21 Nov. 1989; J.G. Rozen and A. Roig leg.; AMNH AMNH_IZC 00290827 .
BOLIVIA • 1 ♂; Chuquisaca Department, E of Muyupampa ; 20 Dec. 1984; L.E. Peña leg.; AMNH AMNH_IZC 00290828 .
BRAZIL • 1 ♀; Rio de Janeiro, Rio de Janeiro (formerly State of Guanabara), Represa Rio Grande ; Jan. 1972; M. Alvarenga leg.; AMNH AMNH_IZC 00290831 .
COSTA RICA • 1 ♀; Alajuela, Peñas Blancas ; 7 Jul. 1981; E. Cruz leg.; RAM • 1 ♀; Cartago, Turrialba ( grounds of the Inter-American Institute for Cooperation on Agriculture ); 3–5 Jun. 1976; M. Wasbauer leg.; EMEC 1135891 About EMEC • 1 ♀; Heredia, La Selva ( 4 km SE of Puerto Viejo) ; 8 May 1980; R. Coville leg.; EMEC 1135892 About EMEC .
ECUADOR • 1 ♀; El Oro, 10 km NE of Piñas ; 7 Jul. 1989; L. Stange and R. Miller leg.; FSCA .
PANAMA • 1 ♂; Darién, Cana Field Station ; 7.7550° N, 77.6850° W; 3 Jun. 1996; J. Ashe and R. Brooks leg.; KUNHM SM0039429 GoogleMaps .
TRINIDAD AND TOBAGO • 1 ♀; Tunapuna-Piarco, Simla Research Station ( 8 km N of Arima) ; 24 Jun.–8. Jul. 1993; S. and J. Peck leg.; CNC 754052 View Materials .
VENEZUELA • 1 ♀; Aragua, Cuyagua ; 14 May??94; AMNH AMNH_IZC 00290829 • 1 ♂; Aragua, El Limon ; 26 Mar. 1987; R. Miller and L. Stange leg.; FSCA • 1 ♀; Aragua, Estación Biológica de Rancho Grande (Portachuelo Pass) ; 10.3500° N, 67.6833° W; 4 Jun. 1998; J. Ashe, R. Brooks and R. Hanley leg.; BOLD sample ID: CCDB-28315 D02; KUNHM SM0124173 GoogleMaps • 1 ♀; same collection data as for preceding; KUNHM SM0124174 GoogleMaps • 1 ♂; Carabobo, Canoabo ; 21 Aug. 1992; L. Masner leg.; CNC 754053 View Materials • 2 ♀♀; Táchira, Pregonero ( Campamento Siberia hospital ); 10–31 Jul. 1989; S. and J. Peck leg.; CNC 754054 View Materials , 754055 View Materials .
Redescription
Male
MEASUREMENTS. Length 6.2 mm; head length 1.6 mm; head width 1.9 mm; fore wing length 5.5 mm.
INTEGUMENT COLORATION. Black in part, at least partially ferruginous on mandible, labrum, clypeus, supraclypeal area, frontal area, antenna, pronotal collar, pronotal lobe, tegula, axilla, mesoscutum, mesoscutellum, metanotum, mesopleuron, metapleuron, propodeum, legs, T1, pygidial plate and metasomal sterna. Mandible with apex darker than rest of mandible; preapical tooth lighter than mandibular apex (difficult to see in holotype because mandibles closed; described from non-type specimens). Antenna brown except scape, pedicel and F1 extensively orange. F2 with orange spot basally. Pronotal collar, pronotal lobe and tegula pale ferruginous to amber. Wing membrane dusky subhyaline, slightly darker at apex. Legs more extensively reddish orange than brown or black.
PUBESCENCE. Face with tomentum densest around antennal socket. Tomentum slightly sparser on clypeus; upper paraocular and frontal areas and vertexal area mostly exposed. Pronotal collar mostly bare in holotype, but with narrow band of pale yellow short, appressed setae along posterior margin in nontype specimens. Mesoscutum without pale tomentum. Mesopleuron nearly bare, except along margins. Metanotum with tomentum uninterrupted, uniformly off-white. T1 with narrow, pale yellow subapical fascia. T2 with broader, complete pale yellow apical fascia without anterolateral extensions. Metasoma otherwise without fasciae, although T3–T6 basally with inconspicuous bands of sparse pale gray-brown hairs. S4 and S5 with long (>1 MOD), curved coppery to silvery subapical hairs, which are often darker apically.
SURFACE SCULPTURE. Punctures dense, but those of head and mesosoma sparser in some areas, larger, deeper and more distinct. Labrum and clypeus with punctures equally dense (most i≤1d). Small impunctate shiny spot lateral to lateral ocellus. Mesoscutum, mesoscutellum and axilla very coarsely and densely rugose-punctate. Tegula densely punctate mesally (i=1–2d), sparsely punctate (i>2d) to impunctate along margins. Mesopleuron with larger and denser (i≤1d) punctures in upper half than ventrolateral half (i≤2d), interspaces shining (somewhat dull due to tessellate surface microsculpture in multiple non-type specimens). Metasomal terga with punctures very fine, dense (i≈1d), evenly distributed on disc. Pygidial plate with large deep punctures more or less evenly spaced throughout, with interspaces shining.
STRUCTURE. Preapical tooth acute. Labral apex with pair of small denticles (separated by shallow concavity), each preceded by longitudinal carina. Frontal keel strongly raised. Frontal area with pair of discrete sparsely punctate protrusions, interspaces shining; each located near upper mesal margin of compound eye. Head dorsally with pair of protrusions, each located where upper genal area meets vertexal area. Vertexal area strongly convex in frontal view. Scape with greatest length 1.5 × greatest width. F2 noticeably longer than wide (L/W ratio = 1.2). Preoccipital ridge separated from hypostomal carina by about 1.5–2 MOD. Pronotal collar elongate (medial length ~1 MOD), expanded laterally to about 2 × medial length in dorsal view and somewhat concave along anterior margin. Mesoscutellum weakly bigibbous, depressed along posterior margin beneath distinct overhanging ridge produced to pair of posteriorly directed teeth. Axilla large, its lateral margin more than half as long as mesoscutellar width (AL/MSCW ratio = 0.7) and tip extending beyond apex of horizontal dorsal portion of mesoscutellum; axilla with tip distinctly pointed, but unattached to mesoscutellum for less than 2 ∕5 (though more than ⅓) the medial length of axilla (with free portion 2 ∕ 5 its medial length or longer in multiple non-type specimens); axilla with lateral margin crenulate, with large tooth near base, and carinate but relatively straight. Mesopleuron with carina delineating its anterior and lateral surfaces. Fore wing with three submarginal cells. Pygidial plate apically truncate.
Female
Description as for male except for usual secondary sexual characters and as follows: F2 even longer than wide (L/W ratio = 1.4); T5 laterally with long, erect simple setae; T5 with pseudopygidial area lunate, its apex less than twice as wide as medial length, indicated by silvery setae on impressed disc of apicomedial region elevated from rest of tergum; pygidial plate with much smaller punctures; S4 and S5 with straight and much shorter hairs (S5 with apical fimbria of coppery to silvery hairs extending beyond apex of sternum by ~½ MOD).
Distribution
Central America and tropical South America + Trinidad in the Lesser Antilles ( Fig. 7A View Fig ). Based on known records, E. boliviensis appears to be the most widely distributed species of Epeolus in the Neotropics.
Ecology
Host records
Michener (1974) reported that females of E. boliviensis (as Tro. schraderi ) were observed frequently flying about the nests of Ancyloscelis Latreille, 1829 ( Hymenoptera : Apidae : Eucerinae ) and Melitoma Lepeletier & Serville, 1828 ( Hymenoptera : Apidae : Eucerinae ), which they entered, and the earth wall where they were located. It remains to be seen whether either or both of these associations is/are true, but, given the strong association of Epeolus with Colletes , these alternative associations are unexpected to say the least. One member of the ‘ Trophocleptria group’, Epeolus bifasciatus , has been observed on separate occasions (personally and by T. Roulston, personal communication, 2016) in the presence of a single species of Colletes , C. latitarsis Robertson, 1891 , so one would expect similar species of Epeolus to be associated with Colletes as well.
Floral records
The label of one examined voucher specimen indicates a floral association with Coffea L. ( Rubiaceae ).
Remarks
The examined primary type specimen of E. boliviensis bears a label that simply says “Type”, which is presumed to be Heinrich Friese’s original type label. Below it is a label that says “ LECTOTYPUS ” and “A. Roig Alsina, 1989 ”. The lectotype designation cannot be traced to any publication, nor does it seem warranted since Friese’s ( 1908) original description of the species gives no indication that it was described from more than one (male) specimen. Moure et al. ( 2007) refer to this specimen as the holotype, not the lectotype, and in the present study it is also recognized as such.
This species exhibits notable variability in integument coloration, with the following features varying from black to partially or entirely ferruginous among examined specimens: pronotal collar, pronotal lobe, axilla, mesoscutum, mesoscutellum, metanotum, mesopleuron, metapleuron and propodeum. In some examined specimens of this species, the T1 subapical fascia has a pair of anterolateral extensions (longitudinal bands). Michener (1974) indicates that Tro. schraderi (herein synonymized under E. boliviensis ) is probably only a geographic variant of E. variolosus (Holmberg, 1886) (as Tro. variolosa). This seems unlikely given that in E. variolosus the mesoscutum is consistently red laterally and black medially, the mesopleura are more densely and finely punctate, and T1 has a single broad apical fascia (the remaining terga lack fasciae), whereas in the Tro. schraderi holotype the mesoscutum is entirely black, the mesopleura are more sparsely and coarsely punctate, T1 has a narrow bright to pale yellow subapical fascia and T2 has a single broad apical fascia (the remaining terga also lack fasciae). Although the male holotype of Tro. variolosa was not examined (according to Moure et al. 2007 it is probably lost), two male syntypes (one at the AMNH and one at the ZMB, the latter bears a label that says “ Lectotypus ” and “A. Roig Alsina, 1989”, but the lectotype designation cannot be traced to any publication) of E. unifasciatus Friese, 1908 , a name which Schrottky (1910) established as a junior synonym of E. variolosus , were examined, along with many non-type specimens from Argentina, Bolivia and Brazil (Supplementary File 1). In the holotype of E. boliviensis the mesoscutum is red laterally and black medially, as it is in E. variolosus , but the specimen much more closely resembles the holotype of Tro. schraderi and specimens from the Caribbean, Central America and northern South America in its much smaller size, more slender appearance, more sparsely and coarsely punctate mesopleuron, and presence of an apical fascia on T2.
Although BIN-compliant sequences are presently not available for E. boliviensis , a partial sequence 293 bp in length is available for a female specimen from Venezuela that closely resembles the Tro. schraderi holotype, which does not cluster closely with the only two BIN-compliant sequences available for what is herein considered to be E. variolosus (sequenced female specimens from Argentina resembling the syntypes of E. unifasciatus ) in a NJ tree of sequences>200 bp in length ( minimum distance = 2.8%, Supplementary File 3). Moreover, the morphological differences between the specimens from the Caribbean, Central America and tropical South America understood to be E. boliviensis and those from in and around the Southern Cone that are understood to be E. variolosus are consistent, and therefore the two forms are herein considered to be heterospecific.
| ZMB |
Germany, Berlin, Museum fuer Naturkunde der Humboldt-Universitaet |
| FSCA |
USA, Florida, Gainesville, Division of Plant Industry, Florida State Collection of Arthropods |
| ZMB |
Museum für Naturkunde Berlin (Zoological Collections) |
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
| FSCA |
Florida State Collection of Arthropods, The Museum of Entomology |
| SEMC |
University of Kansas - Biodiversity Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Apoidea |
|
Family |
|
|
Genus |
Epeolus boliviensis Friese, 1908
| Onuferko, Thomas M. 2019 |
Trophocleptria schraderi
| Michener C. D. 1954: 127 |
Epeolus boliviensis
| Friese H. 1908: 88 |