Hyphessobrycon cantoi, Faria & Guimarães & Rodrigues & Oliveira & Lima, 2021
|
publication ID |
https://doi.org/ 10.1590/1982-0224-2020-0102 |
|
publication LSID |
lsid:zoobank.org:pub:AC428471-B633-46EA-A651-3B5638CFEA27 |
|
DOI |
https://doi.org/10.5281/zenodo.11085281 |
|
persistent identifier |
https://treatment.plazi.org/id/82D27CE6-8349-4F17-A58D-745B3B1B76D2 |
|
taxon LSID |
lsid:zoobank.org:act:82D27CE6-8349-4F17-A58D-745B3B1B76D2 |
|
treatment provided by |
Felipe |
|
scientific name |
Hyphessobrycon cantoi |
| status |
sp. nov. |
Hyphessobrycon cantoi , new species
urn:lsid:zoobank.org:act:82D27CE6-8349-4F17-A58D-745B3B1B76D2
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ; Tab. 1 View TABLE 1 )
Hyphessobrycon gr. heterorhabdus . — Guimarães et al., 2018:7–8, 10 ( Brazil, Pará, Santarém, streams União do Vegetal, Sonrisal, and Irurá, lower rio Tapajós basin; genetic distance from Hyphessobrycon heterorhabdus ; as a putative undescribed species).
Holotype. ZUEC 17228 View Materials , 30.2 mm SL, female, Brazil, Pará State, Santarém, stream tributary of Lago Verde, lower rio Tapajós basin, 02°31’19”S 54°54’58”W, J. D. Bogotá-Gregory, 17 May 2015. GoogleMaps
Paratypes. All from Brazil, Pará State, Santarém. Rio Tapajós basin: ZUEC 12437 View Materials , 37 View Materials , 20.8–30.3 mm SL, same locality and collector as holotype, 29 Jan 2015. ANSP 207974 About ANSP , 5 About ANSP , 24.3–29.5 mm SL; FMNH 144981 About FMNH , 5 About FMNH , 24.3–26.4 mm SL; INPA 59495 View Materials , 5 View Materials , 24.8–30.2 mm SL; UF 245654 , 5 , 23.7–27.3 mm SL; ZUEC 12440 View Materials , 136 View Materials , 20.9–33.3 mm SL, 3 C&S, 22.2–27.0 mm SL, same data as holotype. ZUEC 12439 View Materials , 146 View Materials , 13.1–30.6 mm SL, same locality and collector as holotype, 8 Aug 2015. LBP 30214, 10, 12.5–29.5 mm SL, União do Vegetal, rio Tapajós GoogleMaps basin, 02°28’50.10”S 54°47’19.10”W, L. R. R. Rodrigues, 27 Sep 2020. MPEG 35994 View Materials , 4 View Materials , 15.9–27.4 mm SL, comunidade Cucurunã, igarapé Cucurunã, 02°28’54”S 54°46’13”W, M. Sudário, 12 May 2017. MPEG 35996 View Materials , 14 View Materials , 18.7–28.8 mm SL, same locality as previous, M. Sudário, 21 Apr 2017. UFOPA 703 , 25 , 10.4–28.6 mm SL, igarapé Irurá, 02°27’50”S 54°44’6”W, D. Franco & F. Ribeiro, 1 May 2012. UFOPA 723 , 21 , 13.5–31.9 mm SL, igarapé Irurá, 02°29’23”S 54°44’16”W, D. Franco & F. Ribeiro, 11 May 2012. UFOPA 776 , 51 , 12.3–25.1 mm SL, igarapé Irurá, 02°27’32”S 54°44’6”W, D. Franco & F. Ribeiro, 1 Sep 2012. UFOPA 885 , 17 , 20.3–32.4 mm SL, igarapé Sonrisal , 02°32’S 54°55”W, T. Torres, C. Silva & J. Souza, 13 Jan 2014. UFOPA 901 , 89 , 14.8 –32.0 mm SL, igarapé Irurama GoogleMaps , 02°29’3”S 54°50’12”W, T. Torres, C. Silva & J. Souza, 15 Jan 2014. UFOPA 932 , 8 , 24.7–32.7 mm SL, igarapé Santa Luzia GoogleMaps , 02°32’37”S 54°52’8”W, T. Torres, C. Silva, J. Souza, A. Canto & F. Ribeiro, 18 Jan 2014. Rio Amazonas basin: ZUEC 14597 View Materials , 46 View Materials , 20.2–32.1 mm SL, Mararu, igarapé do Diamantino GoogleMaps , 02°30’16”S 54°39’33”W, T. C. Faria & K. L. A. Guimarães, 9 Aug 2018.
Diagnosis. Hyphessobrycon cantoi can be distinguished from all congeners, except H. amapaensis , H. ericae , H. heterorhabdus , H. sateremawe and H. wosiackii , by the presence of an elongated, anteriorly well-defined humeral blotch that becomes progressively diffuse and blurred posteriorly, overlapping with a midlateral dark stripe. Hyphessobrycon cantoi can be distinguished from H. ericae and H. wosiackii by lacking a caudal peduncle blotch (vs. presence of a caudal peduncle blotch). Hyphessobrycon cantoi can be distinguished from H. amapaensis , H. heterorhabdus and H. sateremawe by lacking a ventral extension of the humeral blotch (vs. ventral extension of the humeral blotch present, although absence of ventral extension may occurs in specimens of H. amapaensis ). Hyphessobrycon cantoi can be further distinguished from H. amapaensis by presenting a conspicuous midlateral dark stripe (vs. inconspicuous midlateral dark stripe) and by possessing a relatively thin red longitudinal stripe (vs. midlateral red stripe very thick and conspicuous). Hyphessobrycon cantoi can be also further distinguished from H. sateremawe by presenting a humeral blotch narrower, occupying vertical height equivalent to less than one scale row to middle of body (vs. humeral blotch and continuous midlateral stripe broad, occupying vertical height equivalent of two scale rows to middle of body). Hyphessobrycon cantoi is also distinguished from H. heterorhabdus by>9% of genetic distance in the cytochrome c oxidase I ( COI) gene. Hyphessobrycon cantoi can be distinguished from H. heterorhabdus by 53–79 mutations and from H. ericae by 87 mutations in the COI gene (S3).
Description. Morphometric data of holotype and paratypes in Tab. 1 View TABLE 1 . Body compressed. Greatest body depth at vertical through dorsal-fin origin. Dorsal profile of head slightly convex from upper lip to vertical through posterior nostril, straight from that point to tip of supraoccipital spine. Dorsal profile of body slightly convex from latter point to dorsal-fin origin. Dorsal-fin base straight, posteroventrally slanted, slightly convex from end of dorsal fin base to adipose-fin insertion and slightly concave between adipose-fin insertion and origin of anteriormost dorsal procurrent caudal-fin ray. Ventral profile of head and body convex from anterior tip of lower jaw to anal-fin origin. Anal-fin base straight, posterodorsally slanted. Ventral profile of caudal peduncle slightly concave.
Jaws equal, mouth terminal. Posterior terminus of maxilla reaching vertical through anterior margin of iris. Maxilla approximately at 45 degrees angle relative to longitudinal axis of body. Nostrils close to each other, anterior opening oval, posterior opening crescent-shaped. Premaxillary teeth in two rows. Outer teeth row with 2(20), 3(20), or 4(2) conic teeth. Inner row with 5(41) bi- to tetracuspid teeth, symphyseal tooth narrower than remaining teeth. Maxilla with 2(1), 3(1), or 4(1) conical to tricuspid teeth. Dentary with 9(1), 10(1) or 11(1) teeth, anteriormost 3–4 teeth larger, tricuspid, 6 remaining teeth considerably smaller and conical. Central cusp of all teeth more developed than remaining lateral cusps.
Scales cycloid. Two to seven parallel radii strongly marked, circulii well marked anteriorly, on covered portion, weakly marked posteriorly. Lateral line slightly deflected downward and incompletely pored, with 7(4), 8(26), 9(16), 10*(4), 11(3) or 13(1) perforated scales. Longitudinal scales series including lateral-line scales 31(2), 32(6), 33*(22), 34(13), 35(7) or 36(2). Longitudinal scale rows between dorsal-fin origin and lateral line 5*(58) or 6(2). Longitudinal scale rows between lateral line and pelvic-fin origin 3*(49) or 4(1). Predorsal scales 9(13), 10*(37), 11(9) or 12(1). Circumpeduncular scales 11(1) or 12*(58). Caudal fin with few small scales basally.
Dorsal-fin rays ii,8(2), 9*(57) or 10(1). Dorsal-fin origin slightly anterior of middle of standard length. First dorsal-fin pterygiophore inserting behind neural spine of 9 th (3) vertebrae. Adipose fin present. Anteriormost anal-fin pterygiophore inserting posterior to haemal spine of 14 th (1) or 15 th (2) vertebrae. Anal-fin rays iv,17*(13), 18(30), 19(15) or 20(2). Last unbranched and first to third anteriormost branched rays distinctly longer than remaining rays, subsequent rays gradually decreasing in size. Pectoral-fin rays i,9(6), 10*(38), 11(15) or 12(1). Pelvic-fin rays i,6(9) or 7*(51). Tip of pelvic fin reaching anteriormost anal-fin rays. Caudal fin forked, lobes roughly rounded and of similar size. Nine (1) or 11(2) dorsal procurrent caudal-fin rays, and 7(1) or 8(2) ventral procurrent caudal-fin rays. Vertebrae 32(1) or 33(2). Supraneurals 4(3), upper portion wider. Branchiostegal rays 4. First gill arch with 2(2) or 3(1) hypobranchial, 6(1) or 8(2) ceratobranchial, 1(3) on cartilage between ceratobranchial and epibranchial, and 6(3) epibranchial gill-rakers.
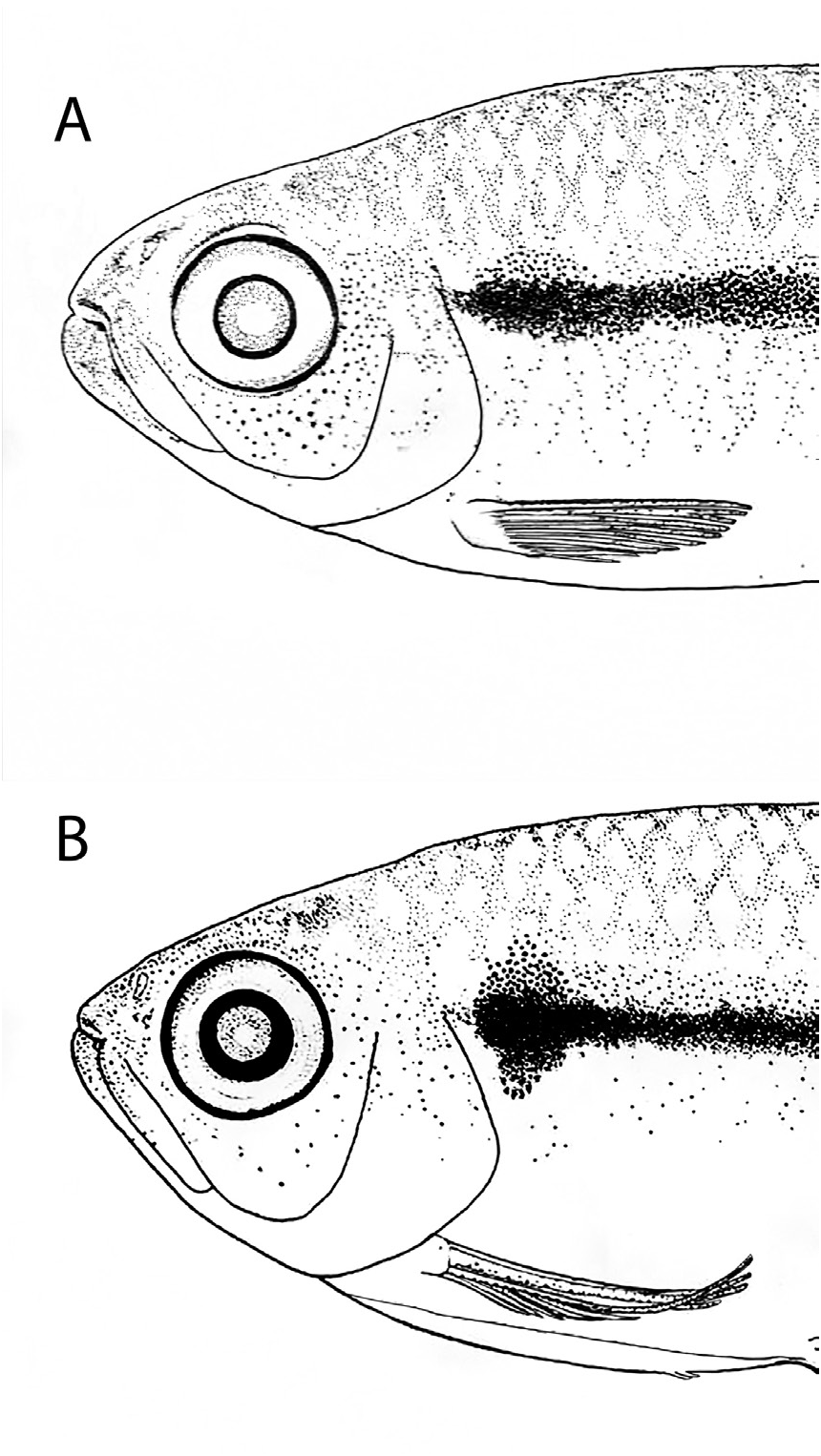
Color in alcohol. Overall body color beige ( Fig. 1 View FIGURE 1 ). Dorsal portion of head and body darker. Ventral portion of head and body with few scattered dark chromatophores. Snout and lower lip dark. Dark chromatophores scattered across infraorbitals and opercle, concentrated dorsally. Predorsal and preadipose scales with conspicuous central dark blotches. Three dorsalmost scale rows with conspicuous reticulated pattern formed by dark chromatophores concentrated at scales margins. Area immediately above humeral blotch and midlateral dark stripe clear, with evenly-scattered, light-grey chromatophores. Humeral blotch narrow and conspicuous. Anterior region well defined, horizontally elongated, with diffuse dorsal expansion and ventral margin approximately straight to slightly convex, becoming gradually diffuse posteriorly, thicker in some specimens. Posterior region of humeral blotch gradually becoming diffuse and coalescing with midlateral dark stripe. Midlateral dark stripe thicker anteriorly, narrower and more diffuse posteriorly, reaching caudal peduncle. Ventral half of abdominal region clear, with few, evenly-scattered chromatophores. Region above anal fin darker, with concentration of dark chromatophores increasing towards caudal peduncle. Dark chromatophores aligned along myocommata of hypaxial muscles above anal fin or above posterior half of anal fin. Caudal fin mostly hyaline, with high concentration of dark chromatophores on middle caudal rays. Anal fin mostly hyaline, with dark chromatophores scattered along interradial membranes, more concentrated along distal region of anal-fin lobe. Dark chromatophores line parallel to anal-fin base. Dorsal-fin rays mostly hyaline, with dark chromatophores concentrated on anteriormost rays. Adipose fin with few scattered dark chromatophores mainly concentrated on proximal region. Pectoral and pelvic fins with dark chromatophores scattered.
Color in life. Based on a picture provided by A. L. C. Canto ( Fig. 2 View FIGURE 2 ), and pictures taken by one of the authors ( LRRR). Overall body color clear, with olivaceous hue. Lower half of head and abdominal region silvery. Dorsal portion of eye red. Tricolor longitudinal pattern starting immediately posterior to opercle and ending immediately before base of caudal fin, composed of narrow dorsal red stripe, narrow middle iridescent stripe and narrow ventral dark longitudinal pattern composed of humeral blotch followed posteriorly by midlateral stripe. Longitudinal red stripe anteriorly continuous, becoming row of red spots at midbody. Middle stripe golden, relatively narrow and well defined, thicker anterior to humeral blotch, becoming row of golden spots at midbody. Scattered, iridescent greenish chromatophores immediately ventral to humeral blotch and anterior region of midlateral stripe.
Sexual dimorphism. Last unbranched and two anteriormost branched anal fin rays larger in females, resulting in a slightly more pointed and developed anal-fin lobe ( ZUEC 12440; ZUEC 14597). Males present tiny bony hooks on distal half of last unbranched anal-fin ray and all branched anal-fin rays (5 to 44), on caudal-fin rays (0 to 8), on distal half of dorsal-fin rays (0 to 30), on all extension of pelvic-fin rays (7 to 40) and on distalmost region of pectoral fin (0 to 12) (all bony hook counts made in a single C&S specimen, ZUEC 12440, 25.2 mm SL). Bony hooks larger on anteriormost rays of anal fin and on pelvic fin ( ZUEC 12440, 65, 21.0– 26.7 mm SL; ZUEC 14597, 5, 20.3–24.8 mm SL). Bony hooks of dorsal, caudal and pectoral fins only discernible in C&S individuals. Smaller male presenting bony hooks 20.3 mm SL ( ZUEC 12440). Females reach larger sizes than males (largest female examined with 33.3 mm SL and largest male with 27.8 mm SL).
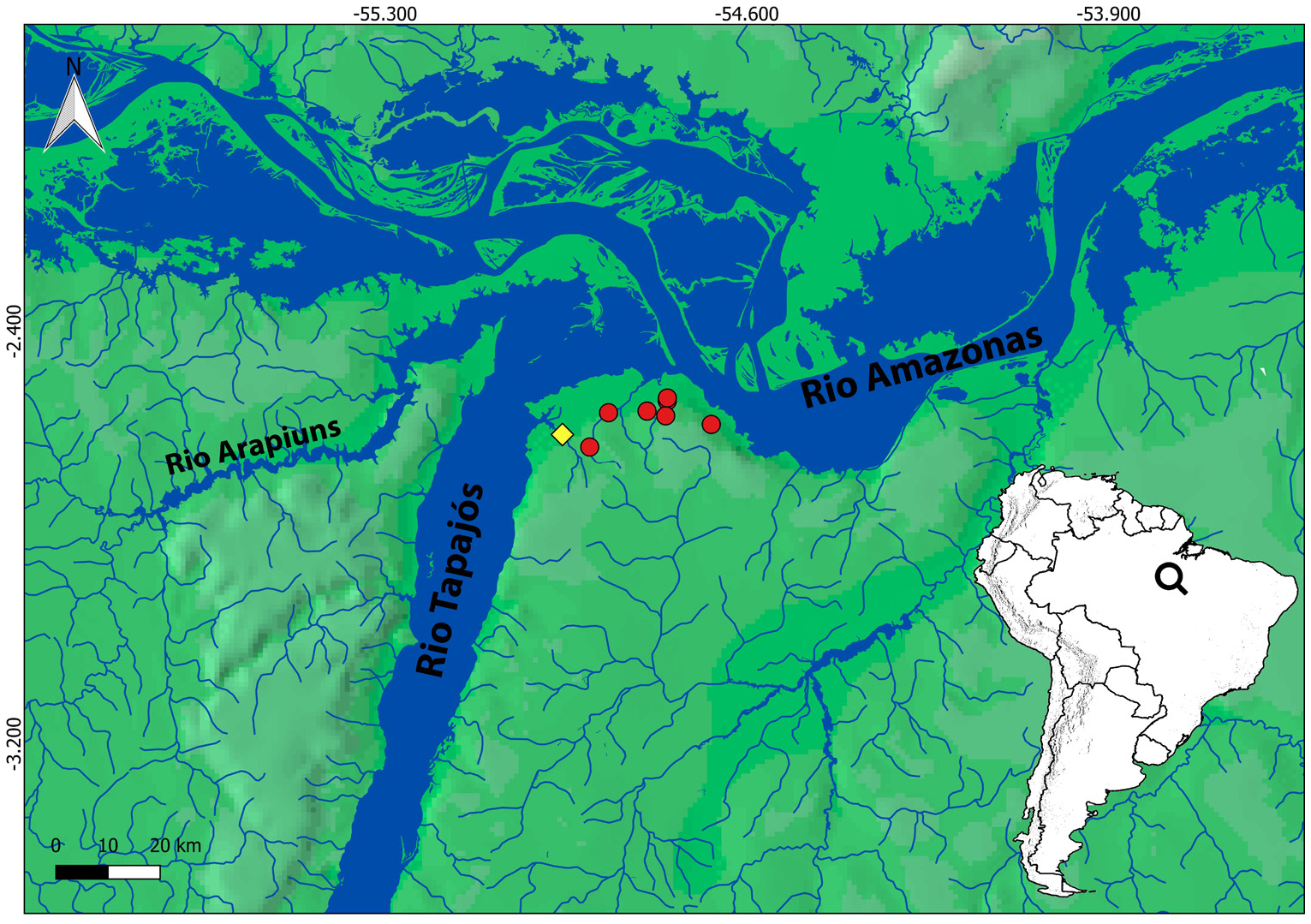
Geographical distribution. Hyphessobrycon cantoi is known from streams draining into the rio Tapajós mouth, and in streams immediately eastward draining directly into the rio Amazonas, state of Pará, Brazil ( Fig. 3 View FIGURE 3 ).
Ecological notes. Hyphessobrycon cantoi is known from slow-flowing, clear water streams. Specimens collected from Igarapé do Diamantino, Santarém, state of Pará, were observed in schools with up to 25 individuals, typically swimming among floating leaves of aquatic plants ( Nymphaea sp. or Nymphoides sp. ). Syntopic species were Copella callolepis (Regan, 1912) , Crenuchus spilurus Gunther, 1863 , and Nannostomus marginatus Eigenmann, 1909 . Another locality from where the species is known (a stream tributary of Lago Verde) presented the following range of physico-chemical parameters across a period of 10 months (November-August): temperature 26.4–27.6ºC, pH 4.3–5.2, dissolved oxygen 3.6–7.9, conductivity 8.52–13.12, and turbidity 4.22–13.3 (Juan D. Bogotá-Gregory, 2020, pers. comm.). Gut content of 3 C&S individuals ( ZUEC 12440) revealed the presence of ants, beetles, Diptera larvae, a Trichoptera larvae, unidentified arthropods remains and unidentified organic matter.
Etymology. The specific name is a homage to André Luiz C. Canto, curator of the fish collection of the Universidade Federal do Oeste do Pará ( UFOPA), in recognition of his contribution to the knowledge of the fishes from the rio Tapajós basin. A genitive noun.
Conservation status. Hyphessobrycon cantoi is only known from streams in the lower Tapajós and nearby streams emptying directly into the rio Amazonas, with an Extent of Occurrence ( EOO) of 134.9 km ² in Santarém. Some of the streams where the species was collected lie at periurban areas, and consequently are under some anthropogenic disturbance, i.e., deforestation, and domestic sewage disposal. The type locality and some of the collection sites of the species lie within the Área de Proteção Ambiental Alter do Chão , which gives them some level of protection against environmental degradation, but even these areas are currently under real state pressure and human induced wildfires. Considering the small EOO and expected increase in degradation of many of the streams from where the species is currently known in the coming years due to the expansion of the urban area of Santarém , we recommend Hyphessobrycon cantoi to be considered Near Threatened ( NT) due to its closeness to fulfilling the criteria as Vulnerable ( VU) B2 biii following the International Union for Conservation of Nature ( IUCN) rules (2019) .
Remarks. Variation on the humeral blotch thickness was observed in populations of Hyphessobrycon cantoi , with thicker humeral blotches occurring in populations of the westernmost portion of the distribution of the species (Lago Verde drainage, rio Tapajós basin) in comparison with population of the easternmost portion of the distribution (igarapé do Diamantino, rio Amazonas basin). Specimens from the Lago Verde drainage also present fewer chromatophores on the abdominal region when compared to the easternmost populations, where, in some individuals, a subtle reticulated pattern can be discerned (compare Figs. 1 View FIGURE 1 A-B).
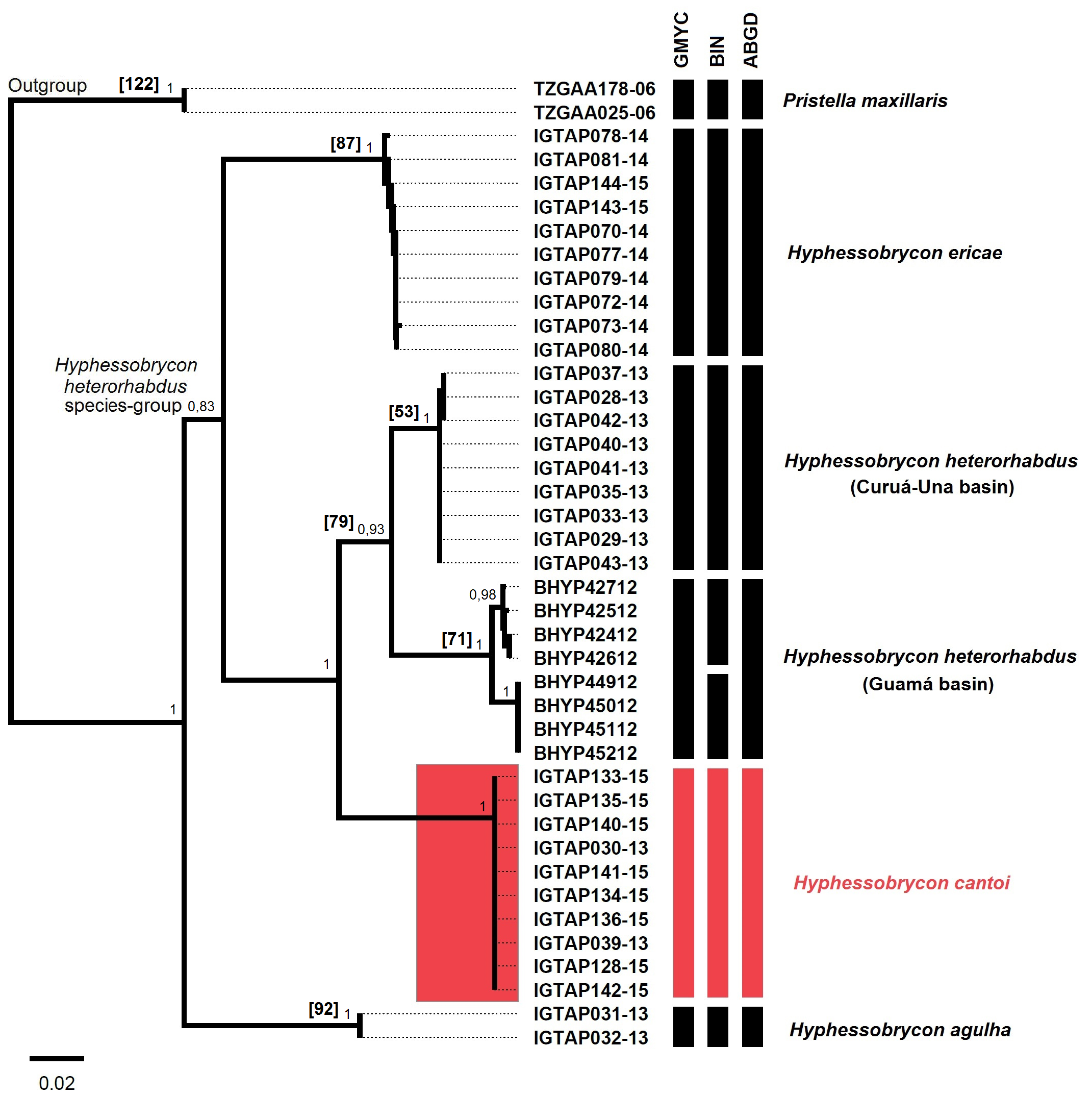
Molecular species delimitation. We analyzed 37 COI sequences (DNA barcoding) belonging to the Hyphessobrycon heterorhabdus species-group from the Tapajós, Curuá-Una and Guamá basins. The sequences were 602bp long and revealed 121 polymorphic sites, base composition of T (31.9%), C (26.5%), A (23.0%), G (18.5%). We did not observe stop codons and indels. The Neighbor-joining reconstruction clearly delimited the H. heterorhabdus species-group (Bootstrap = 100%) as sister group to H. agulha and have H. cantoi aligned with H. heterorhabdus and H. ericae ( Fig. 4 View FIGURE 4 ). The three methods used to delimit species based on COI sequences were congruent to illuminate the molecular identity and the specific status of H. cantoi , which diverged from its congeners by 9.6 to 17.8% ( Tab. 2 View TABLE 2 ). Additionally, we observed two clusters within H. heterorhabdus that segregate individuals from rio Curuá-Una and rio Guamá basins by 6.4% COI divergence, and H. agulha diverged from the H. heterorhabdus species-group by 14.8 to 18.6%.
| R |
Departamento de Geologia, Universidad de Chile |
| T |
Tavera, Department of Geology and Geophysics |
| COI |
University of Coimbra Botany Department |
| ZUEC |
Museu de Zoologia da Universidade Estadual de Campinas |
| SL |
University of Sierra Leone, Njala University College |
| NT |
Department of Natural Resources, Environment and the Arts |
| VU |
Voronezh State University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |