Ranitomeya sirensis Aichinger 1991
|
publication ID |
https://doi.org/10.11646/zootaxa.3083.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/1D338788-9507-1511-C8FC-9E4B3F12F983 |
|
treatment provided by |
Felipe (2021-08-23 20:40:41, last updated by Plazi 2023-11-04 13:58:37) |
|
scientific name |
Ranitomeya sirensis Aichinger 1991 |
| status |
|
Ranitomeya sirensis Aichinger 1991 View in CoL
Account authors: J.L. Brown, E. Twomey, M. Pepper, M. Sanchez-Rodriguez, P.R. Melo-Sampaio, M.B. Souza Figs. 3 View FIGURE 3 , 4 View FIGURE 4 , 9 View FIGURE 9 , 25 View FIGURE 25 (j – t), 26 (a – p), 27 (a – f), 28, 31
Tables 1 – 6
Dendrobates quinquevittatus View in CoL (non Steindachner 1864)—Silverstone 1975: p. 35 (partim) [Huánuco, Tingo Maria (CAS 85147, 85150; USNM 1666904-06)]; Meede 1980: p. 39
Dendrobates ventrimaculatus View in CoL (non Shreve 1935)— Caldwell & Myers 1990 (partim): p. 19, Fig. 12 View FIGURE 12 [ USNM 268841 – 268844 About USNM collected by Reginald B. Cocroft at Tambopata, Madre de Dios, Peru]
Dendrobates sirensis Aichinger 1991: p. 1 View in CoL , Fig. 1 – 3, Table 1 [NHMW 31892 (holotype) collected by Manfred Aichinger in the Serrania de Sira, Río Llullapichis drainage, Huánuco, Peru, 1987];— Schulte 1999: p. 135, Fig. DB-075; Santos et al. 2009, by implication
Dendrobates biolat Morales 1992 View in CoL ; p. 195, Table 2, 3, Figs. 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 [MUSM 7143 (holotype) collected by Victor R. Morales at the Reserva de la Biosfera del Manu, Madre de Dios, Perú, 1987]; – Duellman & Thomas 1996; Schulte 1999: p. 121, Fig. DB-071; De La Riva et al. 2000: p. 3; Doan & Arriaga 2002: p. 108; Lötters et al. 2003: p. 1908; Christmann 2004: p. 6, Figs. on p. 37, 159; Medina-Müller 2006: p. 1; Roberts et al. 2006a: p. 381, Table 1, Figs. 1, 4 View FIGURE 4 ; Brown et al. 2008a: p. 5, Fig. 1; Santos et al. 2009, by implication
Dendrobates lamasi Morales 1992 View in CoL ; p. 191, Fig. 1, 2 View FIGURE 2 , 5 View FIGURE 5 Table 1, 2 [MUSM 1461 (holotype) collected by Victor R. Morales at Bosque Castilla, northwest of Iscozacín, Huanacabamba, Pasco, Peru, 1986];— Schulte 1999: p. 115, Figs. PB-050, PB-018; Lötters et al. 2003: p. 1909; Symula et al. 2003: p. 453, Table 1, Figs. 2–6 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 ; Christmann 2004: p. 6, Figs. on p. 34, 35, 129–132, 148–149, 155– 157; Roberts et al. 2006a: p. 381, Table 1, Fig. 4 View FIGURE 4 ; Santos et al. 2009, by implication
Ranitomeya biolat View in CoL — Grant et al. 2006: p. 171, Fig. 76; Lötters et al. 2007: p. 466, Figs. 582–586; Maldonado et al. 2007: p. 14, Fig. 1; von May et al. 2008a: p. 395, Appendix 2, 2008b: p. 66, Table 1, Fig. 1; Waldram 2008: p. 232, Fig. 1-2; Melo-Sampaio & Souza 2009: p. 447
Ranitomeya lamasi View in CoL — Grant et al. 2006: p. 171, Fig. 76; Lötters et al. 2007: p. 484, Figs. 610–618; von May et al. 2008a: p. 396, Appendix 2
Ranitomeya sirensis View in CoL — Grant et al. 2006: p. 171; Lötters et al. 2007: p. 513, Figs. 614–642; von May et al. 2008a: p. 394, Appendix 1
Background information. This species was described in 1991 (as Dendrobates sirensis View in CoL ) on the basis of four specimens collected in the 1970s and 1980s from the western slope of the northern Cordillera El Sira (also known as the Serranía de Sira). This site was the focus of several scientific investigations during the 1960s and 1970s, including one herpetological survey conducted in 1973 (Duellman and Toft 1979). Surprisingly, this survey did not encounter any Ranitomeya species and it was not until 1976 that this species was first collected, found in elfin forest at 1,560 m elevation by Werner Hanagarth. Later, in the mid 1980s, Manfred Aichinger led a herpetological survey in the Sira which lasted over a year. During this survey, only five individuals were encountered, two of which escaped after capture.
Recent efforts have been made to sample this interesting species. Not only was this species described from a remote and isolated mountain range, but morphologically it seemed quite distinct from other Ranitomeya species , lacking the limb reticulation typical of this genus. In 2005, M. Pepper and M. Sanchez-Rodriguez led an expedition to the Sira in an attempt to find R. sirensis . They were able to reach one of Aichinger’s camps but were unable to locate any R. sirensis . In 2007, Pepper and Sanchez-Rodriguez returned to the Sira with J.L. Brown and E. Twomey and were lucky enough to find two adult R. sirensis less than 4 kilometers from the type locality at an elevation of 442 m (an elevation much lower than previously known for this species—it remains questionable whether this species occurs at elevations> 1400 m, as stated in the original description). This discovery occurred while returning to the camp after searching higher elevation forests less than 1 km from the type locality. The two frogs perfectly matched the original description (pictured in Fig. 26l, n View FIGURE 26 ) and were wedged deep within the axil of a Xanthosoma plant on an abandoned hunting camp.
Later that year J.L. Brown sequenced samples collected from toe-clips of these individuals and was surprised to find the sequences nested within a clade of Ranitomeya lamasi , described by Morales (1992), from Panguana. This clade was part of a larger clade composed of other R. lamasi and R. biolat , also described by Morales (1992). Not satisfied with the knowledge gained from this expedition, Pepper and Sanchez-Rodriguez returned again in 2008 to further clarify the status of this species. When previously in the Sira, the researchers ignored the abundant calls of R. lamasi and did not pursue these individuals. However, in light of the close relationship between lamasi and sirensis based on sequence data, the 2008 team pursued all individuals with lamasi -like calls. After several days the team had collected several courting pairs and solitary individuals. Two of the pairs were partaking in “hybridization” events between R. lamasi and R. sirensis . The first observation consisted of a male R. sirensis (SVL 17.3 mm) courting a female R. lamasi (SVL 15.6 mm, Fig. 26h View FIGURE 26 ). After being captured, the pair was held overnight in a plastic bottle (prior to being released) and they bred. The eggs were fertilized and began to develop. The second observation was of a male R. lamasi courting an intermediate female (likely a mix of the two nominal species: it had coloration like R. sirensis with SVL 16.1 mm, though more orange with faint broken black stripes and ventral spotting, Fig. 26m View FIGURE 26 ). Lastly a lone individual with intermediate morphology (as described above) was observed. These data suggest that these interactions are not atypical at the site where we observed these two nominal species. In fact, only the two individuals sequenced lacked any dark pigmentation (of the five frogs with morphologies similar to R. sirensis ). There is the possibility that the two R. sirensis individuals sequenced were also “hybrids” and both were descendants of parents that had undergone maternal introgression (from R. lamasi ) and the two represent natural species. However, given the frequency of hybridization, similar call and the shared ventral spot, we consider it highly likely that sirensis and lamasi represent one species.
In 2007, J.L. Brown spent four days in southern Peru at the CICRA research station located at the confluence of the Río Los Amigos and Río Madre de Dios. There he and R. von May, who had been conducting field work in this site for years, observed a thriving population of R. biolat that occupied primarily (though not exclusively) a large bamboo forest. Individuals in this population used the abundant phytotelmata within the bamboo internodes for tadpole rearing. Not surprisingly, the species used other phytotelmata, at CIRCA research station they found tadpoles in tree holes and in a mature floodplain forest in the Tambopata National Reserve, R. von May observed ten tadpoles in fallen bracts of Iriartea deltoidea (one of the most common large palms in western Amazonia). At both localties adults we observed calling outside of the bamboo forests (see Natural History). This behavior was similar to R. lamasi (i.e., from near Tingo Maria).
At the CICRA research station and Tambopota National Reserve, R. von May and J.L. Brown encountered individuals that were identical in pattern to lowland populations of R. lamasi , which lacked the characteristic cross on the rostrum (i.e. Fig. 25k View FIGURE 25 ). After sequencing several individuals of R. biolat, J.L. Brown found that several individuals were nested within a larger clade containing other R. lamasi ( Fig. 3 View FIGURE 3 ).
To reconcile the monophyly of either species ( R. biolat and R. sirensis ), so that each was a distinct and valid species, numerous populations of R. lamasi would have to be elevated to specific status. In recent years, gene trees have not been congruent with species trees in some studies (particularly mitochondrial gene trees, as used here; see Brown & Twomey 2009 for more info); however, in this case, we have no evidence to suggest that these two species are not simply morphs of a widespread, highly variable species. Within Ranitomeya lamasi sensu Morales (1992) , several populations possess unique morphologies (i.e., Fig. 27e, 27k View FIGURE 27 , now classified as morphs 2, 3 and 5 below). Of those sampled in our phylogeny, none of them are reciprocally monophyletic with respect to other morphs (including R. biolat and R. sirensis ). Thus, using molecular phylogenetics, behavioral, morphological and acoustic characters, we considered R. biolat and R. lamasi to be junior synonyms of R. sirensis . It seems ironic that the junior synonyms of this species have been subjects of considerable scientific study, though they now bear the name of what once was one of the rarest and most enigmatic poison frogs.
Definition and diagnosis. Assigned to the genus Ranitomeya due to the combination of the following characters: adult SVL<20.0 mm, dorsal coloration conspicuous, dorsolateral stripes extend to top of thighs, brightly colored throat, distinctive pale reticulation on limbs and venter, dorsal skin smooth, finger I greatly reduced and shorter than finger II, finger discs II – IV greatly expanded, disc of finger 2 – 2.4 times wider than finger width, thenar tubercle conspicuous, toe discs III – V moderately expanded, toe webbing absent, larval vent tube dextral, maxillary and premaxillary teeth absent. Adults use phytotelmata for reproduction and deposit eggs above phytotelm. Tadpole light gray, ovoid, with irregular markings present late in development. Five principal morphs of this species currently are known: (i) the Sira morph ( Fig. 26l – n View FIGURE 26 ), (ii) the Lamasi morph ( Fig. 27e – f View FIGURE 27 ), (iii) the Divisoria morph ( Fig. 25p – s View FIGURE 25 ), (iv) the Biolat morph ( Fig. 25j – o View FIGURE 25 ) and (v) the Panguana morph ( Figs. 25t View FIGURE 25 , 26a – g, j,k, o, p View FIGURE 26 , 27 View FIGURE 27 a- d). All possess a large, brightly-colored ventral spot (typically yellow) on a bluish to greenish ground color (a possible synapomorphy shared with R. vanzolinii and occasionally R. flavovittata ).
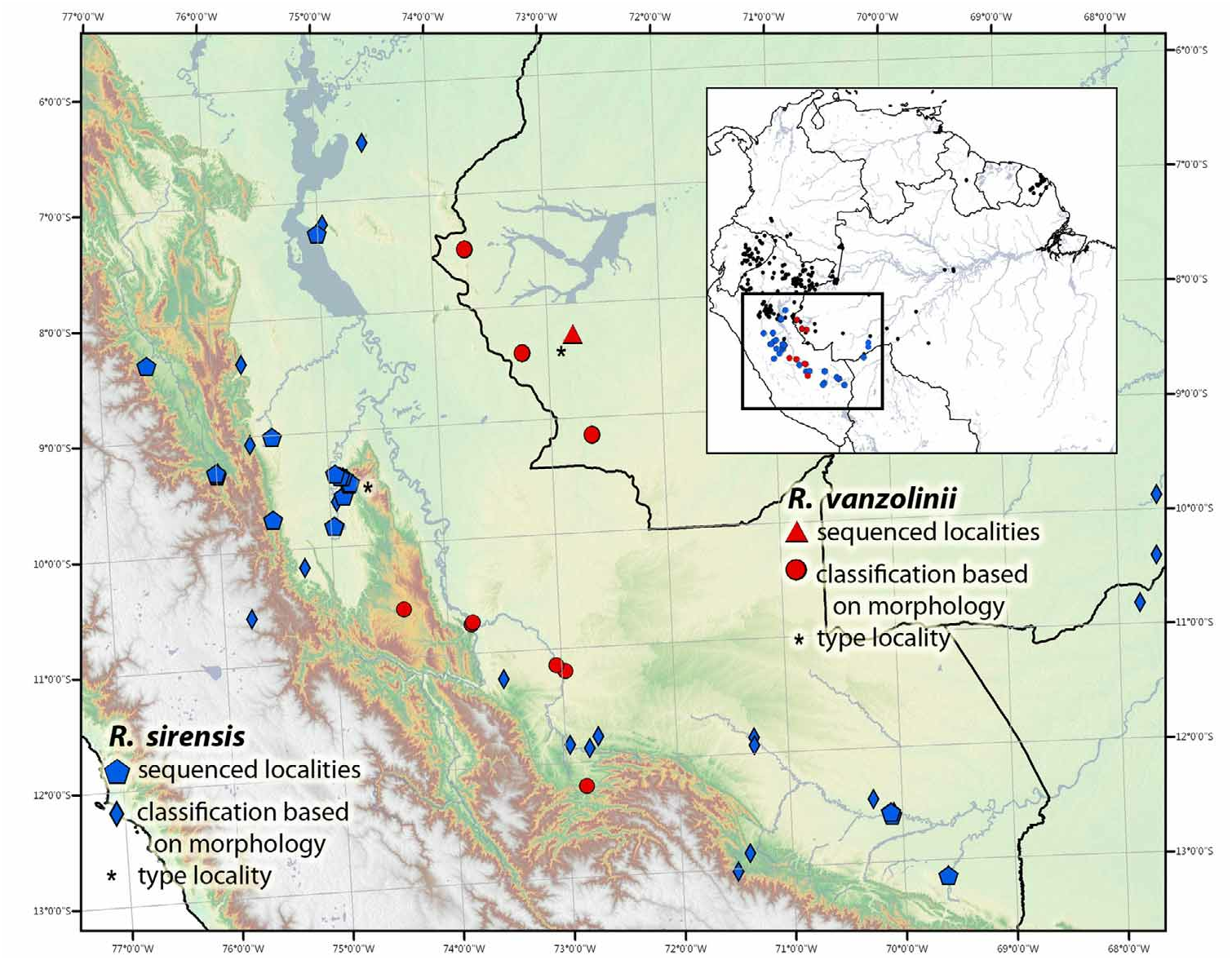
(i) The Sira morph is an amelanistic morph (lacking any black pigmentation). This morph has a solid red to orange-red dorsum and metallic, sage-green limbs and ventral coloration. The throat and center of the belly each have a large spot, identically colored as dorsum. This is the nominotypical morph for the species and is only known to occur within the Cordillera El Sira, Huánuco, Peru.
(ii) The Lamasi morph possesses irregular and broken yellow to yellow-green dorsolateral, ventrolateral and middorsal stripes that often connect to each other irregularly. Venter and limbs are green to blue-green and coarsely reticulated. Most individuals possess bright white to cream spots on the upper surface of the upper thighs, near the groin and upper surface of the forearms, near the axilla. All other morphs have blue to blue-gray reticulation/spotting on venter and limbs on a black ground color. Further, they all possess largely complete middorsal, dorsolateral and ventrolateral stripes. This morph in only known to occur within the lowlands and mid-elevation forests of the Departments Pasco and Huánuco, Peru .
(iii) The Divisoria morph has very broad bright yellow dorsolateral stripes (fusing entirely with oblique lateral stripes) and an identically colored, but thinner middorsal stripe. The middorsal stripe fuses with the labial stripe (anterior to the orbits), creating the appearance of a short ‘Y’ or ‘ T.’ The middorsal stripe is incomplete (though see below); the stripe breaks in the center of the dorsum, and the black ground coloration between the middorsal and dorsolateral stripes creates a large black “X” that covers most of the dorsum (occasionally this morph is referred to as the ‘X’ morph). Other Divisoria populations are identical in all regards; however, they possess a complete middorsal stripe. The black nostril spots fuse on the rostrum, creating the appearance of an upside-down ‘U’ on the tip of the snout. The venter and limbs are typically light blue to sky blue, giving the appearance of being evenly stippled in identically sized large black spots. This morph is only known to occur within the east versant of the Divisoria range of the southern Cordillera Azul near Aguaytia, Department Ucayali, Peru .
(iv) The Biolat morph has thin yellow dorsolateral, middorsal and ventrolateral stripes. A middorsal stripe extends to the tip of the snout, creating the appearance of a crucifix that crosses anterior to orbits. Often the middorsal stripe is slightly darker, appearing yellow-green. The ventrer and limbs are typically blue-green to light gray and can be finely reticulated or evenly stippled in identically sized black spots. This morph occurs predominantly in the lowlands of southern Peru (mostly known from the Department of Madre de Dios and occasionally observed in the Department of Cusco), southwestern Brazil (State: Acre) and northern Bolivia (Department: Pando) where it is typically found in bamboo forests ( Maldonado & Reichle 2007; Melo-Sampaio & Barbosa 2009; von May et al. 2009a).
(v) The Panguana morph is one of the most variable and widespread morphs. The ventral spot, chin, dorsolateral, ventrolateral and middorsal stripes range from bright red, orange, or green to bright or dull yellow. The middorsal stripe fuses with the labial stripe anterior to the orbits, creating the appearance of a short ‘Y’ or ‘ T.’ The black nostril spots typically fuse on the rostrum, creating the appearance of an upside down ‘U’ on the tip of the snout. Most individuals possess large bright white to cream spots on the dorsal surfaces of the limbs at the groin and axilla. The venter and limb coloration is typically bluish green to metallic green to light gray. The limbs and venter can be finely reticulated or have the appearance being evenly stippled in identically sized black spots. This morph occurs within the humid cloud forests and lowland rainforests of Departments Huánuco, Pasco, Junín, Ucayali , southwestern Loreto and southern San Martín.
Morphs 2 – 5 are similar in appearance to R. amazonica , R. toraro sp. nov., R. variabilis , R. ventrimaculata and R. imitator ; however, all these species lack a large, brightly colored belly spot. No other species of Ranitomeya is easily confused with the Sira morph.
Natural history. Recently, von May et al. (2008b) published a detailed description the tadpole of R. sirensis (as Ranitomeya biolat ). Our observations of other populations match this description, based on observations on tadpoles of R. sirensis (the Panguana morph) from Puerto Inca and Contamana.
In that same publication, von May also noted that this species exhibited male-only parental care. This is supported by other observations, i.e., the lack of observations of trophic eggs, which are commonly observed in pools containing R. imitator and R. vanzolinii larvae ( Brown et al. 2008b; Caldwell & de Oliveira 1999). Typically the tadpoles of R. sirensis consumed soft-bodied mosquito larvae ( Trichoprosopon digittatum and Culex sp. ) and occasionally consumed predaceous mosquito larvae in the genus Toxorhynchites ( von May et al. 2009a, b). This species also has considerably larger home ranges than other biparental care species (mean 150 ± 184 m 2 versus 31.50 ± 23.05 m 2 in R. vanzolinii and 10.9 ± 14.31 m 2 in R. imitator ; Waldram 2008, Brown et al. 2009b). However, this comparison is not entirely legitimate because Waldram (2008) used a coarse (and unconventional method) to measure space use, sampling data at a spatial resolution of 25 m 2, a resolution that frequently exceeds the territory sizes of biparental care species and is almost equal to many male-parental care species ( Pröhl 2005; Brown et al. 2009b, Werner et al. 2011). In Tambopata, R. sirensis individuals were observed on average in six grid cells (150 m 2 / 25 m 2 = 6). Because of this, it is fair to assume the home ranges for this species are large (even if the individuals only occurred in a small portion of each grid), and are much larger than those observed for any biparental care species. Ranitomeya sirensis has larger clutches than other biparental care species (3.3 versus 1.6 in R. imitator with biparental care and 3.6 in R. variabilis , a male-only parental care species; Brown et al. 2009b).
Waldram (2008) suspected that tadpole oophagy was an important food source but never observed egg feeding or trophic eggs after six months of observation. Further, von May et al. (2009a, b) studied this species for 12 months and never observed egg feeding or the presence of unfertilized trophic eggs (which are typically deposited singly or in pairs below the surface of the water). In Tambopata, von May observed the tadpoles of R. sirensis cannibalize the tadpoles of Allobates femoralis , Ameerega trivittata , as well as on mosquito larvae, whenever they cooccurred in the same phytotelm. Because Waldram (2008) did not report whether tadpoles were consuming eggs or newly hatched tadpoles, it is difficult to determine if this species actually consumes embryos, as do, for example, Ecuadorian R. variabilis (Summers 1999) and French Guianan R. amazonica ( Poelman & Dicke 2007) .
Vocalizations. Based on extensive field observations and a small handful of recordings, the call of R. sirensis is a typical vanzolinii -group call. The call is a loud trill, with notes 0.9 – 2.2 sec in length, repeated at 6 – 14 notes per minute. Although we have recordings from only a few localities, we have heard calls of this species at every locality included in our phylogeny (including the type locality) and these calls all appear to be very similar.
Distribution. This species occurs in Amazonian rainforests of Bolivia (Department: Pando), Brazil (State: Acre) and Peru (Departments: Cusco, Huánuco, Junín, Loreto, Madre de Dios, Pasco, San Martín, Ucayali), Fig. 31 View FIGURE 31 .
Conservation status. Following the IUCN Red List categories and criteria ( IUCN 2010), we tentatively suggest listing this species as Least Concern (LC). Due to the small range of some morphs, e.g., the Sira morph, they may require additional protection.
Aichinger, M. (1991) A new species of poison-dart frog (Anura: Dendrobatidae) from the Serrania de Sira, Peru. Herpetologica, 47, 1 - 5.
Brown, J. L., Morales, V. & Summers, K. (2008 a) Divergence in parental care, habitat selection and larval life history between two species of Peruvian poison frogs: an experimental analysis. Journal of Evolutionary Biology, 21, 1534 - 1543.
Brown, J. L., Twomey, E., Morales, V. & Summers, K. (2008 b) Phytotelm size in relation to parental care and mating strategies in two species of Peruvian poison frogs. Behaviour, 145, 1139 - 1165.
Brown, J. L., Morales, V. & Summers, K. (2009 b) Home range size and location in relation to reproductive resources in poison frogs (Dendrobatidae): a Monte Carlo approach using GIS data. Animal Behaviour, 77, 547 - 554.
Caldwell, J. P. & Myers, C. W. (1990) A new poison frog from Amazonian Brazil, with further revision of the quinquevittatus group of Dendrobates American Museum Novitates, 2988, 1 - 21.
Caldwell, J. & de Oliveira, V. (1999) Determinants of biparental care in the spotted poison frog, Dendrobates vanzolinii (Anura: Dendrobatidae). Copeia, 565 - 575.
Christmann, S. P. (2004) Dendrobatidae - Poison Frogs - A Fantastic Journey through Ecuador, Peru and Colombia (Volumes I, II & III).
De La Riva, I., Kohler, J., Lotters, S. & Reichle, S. (2000) Ten years of research on Bolivian amphibians: updated checklist, distribution, taxonomic problems, lityerature and iconography. Revista Espanola de Herpetologia, 14, 19 - 164.
Doan, T. M. & Arriaga, W. A. (2002) Microgeographic variation in species composition of the herpetofaunal communities of Tambopata Region, Peru. Biotropica, 34, 101 - 117.
Duellman, W. E. & Thomas, R. (1996) Anuran amphibians from a seasonally dry forest in southeastern Peru and comparisons of the anurans among sites in the Upper Amazon Basin. Occasional Papers of the Natural History Museum, University of Kansas, Lawrence, Kansas, 1 - 34.
Grant, T., Frost, D. R., Caldwell, J. P., Gagliardo, R., Haddad, C. F. B., Kok, P. J. R., Means, D. B., Noonan, B. P., Schargel, W. E. & Wheeler, W. (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia, Athesphatanura, Dendrobatidae). Bulletin of the American Museum of Natural History, 299, 1 - 262.,
IUCN (2010) IUCN Red List Categories and Criteria: Version 8.1. IUCN Species Survival Commission, IUCN, Gland, Switzerland and Cambridge, UK, 85 pp.
Lotters, S., Reichle, S. & Jungfer, K. - H. (2003) Advertisement calls of neotropical poison frogs (Amphibia: Dendrobatidae) of the genera Colostethus, Dendrobates and Epipedobates, with notes on dendrobatid call classification. Journal of Natural History, 37, 1899 - 1911,
Lotters, S., Jungfer, K. - H., Schmidt, W. & Henkel, F. W. (2007) Poison Frogs: Biology, Species and Captive Husbandry Edition Chimaira, Frankfurt am Main, 668 pp.
Maldonado, M. M. & Reichle, S. (2007) Primer registro de Ranitomeya biolat (Morales, 1992) (Anura: Dendrobatidae) para Bolivia, con apuntes sobre la nueve nomenclatura de Dendrobatidae. Kempffinan, 3 (1), 14 - 17.
Medina-Muller, M. (2006) Factores que Influyen en el Canibalismo entre Renacuajos de la Rana Venenosa Dendrobates biolat Morales 1998 (Anura: Dendrobatidae). Tesis, Licenciatura. Universidad Ricardo Palma, Lima, Peru.
Meede, U. (1980) Beobachtungen an Dendrobates quinquevittatus und Phyllobates fermoralis. Salamandra, 16, 38 - 51.
Melo-Sampaio, P. R. & Barbosa de Souza, M. (2009) Ranitomeya biolat. Herpetological Review, 40 (4), 447.
Morales, V. (1992) Dos especies nuevas de Dendrobates (Anura: Dendrobatidae) para Peru. Caribbean Journal of Science, 28, 191 - 199.
Poelman, E. H. & Dicke, M. (2007) Offering offspring as food to cannibals: oviposition strategies of Amazonian poison frogs (Dendrobates ventrimaculatus). Evolutionary Ecology, 21, 215 - 227.
Prohl, H. (2005) Territorial behavior in Dendrobatid Frogs. Journal of Herpetology, 39, 354 - 365.
Roberts, J. L., Brown, J. L., von May, R., Arizabal, W., Presar, A., Symula, R., Schulte, R. & Summers, K. (2006 a) Phylogenetic relationships among poison frogs of the genus Dendrobates (Dendrobatidae): A molecular perspective from increased taxon sampling. Herpetological Journal, 16, 377 - 385.
Santos, J. C., Coloma, L. A., Summers, K., Caldwell, J. P., Ree, R. & Cannatella, D. C. (2009) Amazonian Amphibian Diversity Is Primarily Derived from Late Miocene Andean Lineages. PLoS Biol, 7, e 1000056.
Schulte, R. (1999) Pfeilgiftfrosche Artenteil - Peru . INBICO, Wailblingen, Germany, 294 pp.
Shreve, B. (1935) On a new Teiid and Amphibia from Panama, Ecuador, and Paraguay. Occasional Papers of the Boston Society of Natural History, 8, 209 - 218.
Symula, R., Schulte, R. & Summers, K. (2003) Molecular systematics and phylogeography of Amazonian poison frogs of the genus Dendrobates. Molecular Phylogenetics and Evolution, 26, 452 - 75.
Twomey, E. & Brown, J. L. (2009) Another species of Ranitomeya (Anura: Dendrobatidae) from Amazonian Colombia. Zootaxa, 1302, 48 - 60.
von May, R., Catenazzi, A., Angulo, A., Brown, J. L., Carrillo, J., Chavez, G., Cordova, J. H., Curo, A., Delgado, A., Enciso, M. A., Gutierrez, R., Lehr, E., Martinez, J. L., Medina-Muller, M., Miranda, A., Neira, D. R., Ochoa, J. A., Quiroz, A. J., Rodriguez, D. A., Rodriguez, L. O., Salas, A. W., Seimon, T., Seimon, A., Siu-Ting, K., Suarez, J., Torres, J. & Twomey, E. (2008 a) Current state of conservation knowledge of threatened amphibian species in Peru. Tropical Conservation Science, 1, 376 - 396.
von May, R., Medina - Muller, M., Donnelly, M. A., & Summers, K. (2008 b) The tadpole of the bamboo - breeding poison frog Ranitomeya biolat (Anura: Dendrobatidae). Zootaxa, 1857, 66 - 68.
von May, R., Medina-Muller, M., Donnelly, M. A., & Summers, K. (2009 a) Breeding-site selection by the poison frog Ranitomeya biolat in Amazonian bamboo forests: an experimental approach. Canadian Journal of Zoology, 87, 453 - 463.
Waldram, M. (2008) Breeding biology of Ranitomeya biolat in the Tambopata region of Amazonian Peru. Journal of Herpetology, 42, 232 - 237.
Werner P., Elle, O., Schulte, L., & Lotters, S. (2011) Home range behaviour in male and female poison frogs in Amazonian Peru (Dendrobatidae: Ranitomeya reticulata). Journal of Natural History, 45 (1 - 2), 15 - 27
FIGURE 2. Illustrated guide to morphological terminology. A. Finger and hand morphology: i. Finger I (far left) <Finger II, thenar tubercle (= inner metacarpal tubercle) present (depicted by arrow), and greatly expanded finger discs in Fingers II-IV. Inset depicts Finger I and a thenar tubercle which is clearly visible. Note that in some Ranitomeya this is trait reduced and difficult to view (as in main picture) (Ranitomeya variabilis pictured, inset of R. benedicta). ii. Finger I ≈ Finger II, thenar tubercle absent. (Adelphobates quinquevittatus pictured) iii. Weakly expanded finger discs in Fingers II-IV (Excidobates captivus pictured). B. Stripes: i. Middorsal (follows vertebral column), dorsolateral (extends from eye to either upper thigh, as pictured, or to vent), ventrolateral (running from groin to axilla) and labial stripe (stripe that extends from shoulder around upper lip)(R. sirensis pictured). ii.. Oblique lateral stripe (extends from groin to eye, as in picture stripe is incomplete anteriorly). Unlabeled arrow depicts a dorsolateral stripe that does not reach thigh, a characteristic of certain species of Andinobates (type ‘A’ in Grant et al. 2006). (Andinobates claudiae pictured). C. Limb patterns: i. Distinct limb reticulation/spotting (characteristic of most species of Ranitomeya) (R. variabilis pictured). ii. Wavy stripes (not classified as distinct limb reticulation) (R. summersi pictured). iii. Patternless. Typical of most Andinobates species (R. sirensis pictured). D. Diagnostic head patterns: i. Large black “oval” on head (R. imitator pictured). ii. Large black “pentagon” or “five-point star” on head (R. summersi pictured). iii. Black band across head entirely covering eyes (known only in a single population of this species near the Pongo de Manseriche, Peru) (R. fantastica pictured). E. Nose spots. i. Two nose spots (R. imitator pictured). ii. Single nose spot. (R. variabilis pictured). iii. Frontward-turned “U” on the tip of snout. (R. toraro pictured). F. Geographical distribution. West: distribution within Andes, west of Andes, or in Central America. East: distribution east of Andes (including Guiana Shield) or in east-Andean versant. G. Dorsal patterns: i.“Y-shape”. Space between stripes create black pattern which forms a black Y on the back. (R. variabilis pictured). ii. Merging of the obliquelateral and dorsolateral stripes (R. variabilis pictured). iii. Broken dorsolateral stripes (R. flavovittata pictured). iv. Spotting (R. imitator pictured). H. Key ventral characters: i. Distinctive throat coloration and ventral reticulation (also shown in H-ii & H-iii) (R. reticulata pictured). ii. Belly patch (R. sirensis pictured). iii. Gular spots (single or paired dark spots at corner of mouth) (R. amazonica pictured). iv. Marbled pattern (not classified as reticulation) (Andinobates virolinensis pictured).
FIGURE 3. A consensus Bayesian phylogeny based on 1011 base pairs of aligned mitochondrial DNA sequences of the 12S (12s rRNA), 16S (16s rRNA) and cytb (cytochrome-b gene) regions. Thickened branches represent nodes with posterior probabilities 90 and greater, other values are shown on nodes. Taxon labels depict current specific epithet, number in tree, the epithet being used prior to this revision (contained in parentheses), and the collection locality. A. Top segment. B. Middle segment. C. Bottom segment of phylogeny.
FIGURE 4. Putative species tree for Andinobates, Excidobates, and Ranitomeya. Placement of species where molecular data were lacking (A. altobueyensis, A. viridis, A. abditus, A. daleswansoni and R. opisthomelas) was based on morphology. Andinobates altobueyensis and A. viridis were placed as sister taxa due to the absence of dark pigmentation on dorsal body and limbs and overall similar dorsal coloration and patterning. These species were placed as sister to A. fulguritus (sequenced) on the basis of similar dorsal coloration (bright green to greenish-yellow). Andinobates opisthomelas was placed in the bombetes group in a polytomy with A. bombetes and A. virolinensis (both sequenced) due to their similar advertisement calls and morphology, particularly their red dorsal pattern and marbled venter. Andinobates daleswansoni was placed as sister to A. dorisswansonae due to the absence of a well-defined first toe in both species. Andinobates abditus was placed in the bombetes group based on a larval synapomorphy which appears to be diagnostic of that group (wide medial gap in the papillae on the posterior labium). However, A. abditus was placed as the sister species to all other members of the bombetes group due to the absence of bright dorsal coloration and isolated geographic distribution. Andinobates abditus is currently the only species of its genus known to occur in the east-Andean versant, thus its placement remains speculative until molecular data become available. Photo credits: Thomas Ostrowski, Karl-Heinz Jungfer, Victor Luna-Mora, Giovanni Chaves-Portilla.
FIGURE 5. Andinobates Plate 1. minutus group: A–G: Andinobates claudiae and habitat (all from Bocas del Toro, Panama. Photos T. Ostrowski); A & B: Buena Esperanza; C–F: Isla Colon; G: Cerro Brujo; H: tadpole in phytotelm; I: habitat in Bocas del Toro, Panama. J–M: Andinobates minutus (all from Colombia. Photos DMV unless noted): J & K: Buenaventura, Valle del Cauca; L: Quibdó, Chocó; M: Baudó, Chocó (photo J. Mejía-Vargas). fulguritus group: N–V: Andinobates fulguritus (all from Colombia, photos DMV unless noted): N: Baudó, Chocó (photo J. Mejía-Vargas); O: Playa de Oro, Chocó (type locality); P–R: Uraba, Chocó. S–V: Anchicayá, Valle del Cauca. (nΦ = number of individual in phylogeny, Ω = population sampled in phylogeny).
FIGURE 9. Known elevation distributions of Ranitomeya. Dotted line is mean for all samples. Dark boxes display the total elevation range of each species, within each contains a corresponding box plot.
FIGURE 12. Ranitomeya toraro sp. nov. type series. All specimens from two localities in Brazil: Amazonas state, municipality of Castanho, at km 12 on road to Autazes (ca. 40 km south of Manaus) or Scheffer Madeireira on Rio Ituxi, ca. 170 km southwest of Lábrea (labeled with ‡). Top row, from the collections of MPEG (L-R): 13839, 13838 (holotype), 13841, 13840, 13842 and 13037(‡). Bottom row, from the collections of OMNH (L-R): 37441, 37440, 36666 (‡), 37442, 37439, 36667(‡), 37438. Black bar = 20 mm (5 mm increments). Sequenced individuals (number in phylogeny): OMNH 36666 (7), OMNH 37440 (5), OMNH 36667 (6), MPEG 13841 (4)
FIGURE 25. Ranitomeya Plate 8. vanzolinii group: A–I: Ranitomeya imitator Curiyacu, San Martin, Peru (‡). J–T: Ranitomeya sirensis (all from Peru unless noted): J-L: CICRA Station, Madre de Dios (Rio Los Amigos, Ω); M: near Rio Branco, Acre, Brazil (PRMS); N & O: Central Rio Urubamba, Cusco (G. Chavez); P & Q: Tingo Maria, Huánuco (ET, Ω); R: Bamboo forest, R. sirensis often uses the phytotelmata within bamboo for tadpole deposition, Tingo Maria, Huánuco (ET); S: Aguaytía, Ucayali; T: Codo del Pozuzo, Huánuco (20Φ). (nΦ = number of individual in phylogeny, Ω = population sampled in phylogeny, ‡ = genetically sampled, but not included in our phylogeny).
FIGURE 26. Ranitomeya Plate 9. vanzolinii group: A–P: Ranitomeya sirensis (all from Peru): A–G: Puerto Inca, Huánuco (JLB and ET, Ω); H & I: Breeding pair of R. sirensis found in the type locality between the lowland and highland populations, Cordillera El Sira, Huánuco (MSR); J & K: Rio Pachitea, Huánuco (J. Stenicka); L–N: Cordillera El Sira, Huánuco (B. Wilson and JLB, 10-11Φ); O: Rio Pachitea, Huánuco (J. Stenicka, 17Φ); P: Yanayacu Maquia, Ucayali. (nΦ = number of individual in phylogeny Ω = population sampled in phylogeny).
FIGURE 27. Ranitomeya Plate 10. vanzolinii group: A–F: Ranitomeya sirensis (all from Peru): A–D: near Contamana, Loreto (JLB and G. Gagliardi, 1-3Φ); E: uncertain locality, likely Iscozacin, Junin (7 Φ); F: Estación Biológica Paujil, Junin (L. Schulte). (nΦ = number of individual in phylogeny).
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ranitomeya sirensis Aichinger 1991
| Brown, Jason L., Twomey, Evan, Amézquita, Adolfo, Souza, Moisés Barbosa De, Caldwell, Jana- Lee P., Lötters, Stefan, May, Rudolf Von, Melo-Sampaio, Paulo Roberto, Mejía-Vargas, Daniel, Perez-Peña, Pedro, Pepper, Mark, Poelman, Erik H., Sanchez-Rodriguez, Manuel & Summers, Kyle 2011 |
Ranitomeya biolat
| Melo-Sampaio, P. R. & Barbosa de Souza, M. 2009: 447 |
| von May, R. & Catenazzi, A. & Angulo, A. & Brown, J. L. & Carrillo, J. & Chavez, G. & Cordova, J. H. & Curo, A. & Delgado, A. & Enciso, M. A. & Gutierrez, R. & Lehr, E. & Martinez, J. L. & Medina-Muller, M. & Miranda, A. & Neira, D. R. & Ochoa, J. A. & Quiroz, A. J. & Rodriguez, D. A. & Rodriguez, L. O. & Salas, A. W. & Seimon, T. & Seimon, A. & Siu-Ting, K. & Suarez, J. & Torres, J. & Twomey, E. 2008: 395 |
| Waldram, M. 2008: 232 |
| Lotters, S. & Jungfer, K. - H. & Schmidt, W. & Henkel, F. W. 2007: 466 |
| Maldonado, M. M. & Reichle, S. 2007: 14 |
| Grant, T. & Frost, D. R. & Caldwell, J. P. & Gagliardo, R. & Haddad, C. F. B. & Kok, P. J. R. & Means, D. B. & Noonan, B. P. & Schargel, W. E. & Wheeler, W. 2006: 171 |
Ranitomeya lamasi
| von May, R. & Catenazzi, A. & Angulo, A. & Brown, J. L. & Carrillo, J. & Chavez, G. & Cordova, J. H. & Curo, A. & Delgado, A. & Enciso, M. A. & Gutierrez, R. & Lehr, E. & Martinez, J. L. & Medina-Muller, M. & Miranda, A. & Neira, D. R. & Ochoa, J. A. & Quiroz, A. J. & Rodriguez, D. A. & Rodriguez, L. O. & Salas, A. W. & Seimon, T. & Seimon, A. & Siu-Ting, K. & Suarez, J. & Torres, J. & Twomey, E. 2008: 396 |
| Lotters, S. & Jungfer, K. - H. & Schmidt, W. & Henkel, F. W. 2007: 484 |
| Grant, T. & Frost, D. R. & Caldwell, J. P. & Gagliardo, R. & Haddad, C. F. B. & Kok, P. J. R. & Means, D. B. & Noonan, B. P. & Schargel, W. E. & Wheeler, W. 2006: 171 |
Ranitomeya sirensis
| von May, R. & Catenazzi, A. & Angulo, A. & Brown, J. L. & Carrillo, J. & Chavez, G. & Cordova, J. H. & Curo, A. & Delgado, A. & Enciso, M. A. & Gutierrez, R. & Lehr, E. & Martinez, J. L. & Medina-Muller, M. & Miranda, A. & Neira, D. R. & Ochoa, J. A. & Quiroz, A. J. & Rodriguez, D. A. & Rodriguez, L. O. & Salas, A. W. & Seimon, T. & Seimon, A. & Siu-Ting, K. & Suarez, J. & Torres, J. & Twomey, E. 2008: 394 |
| Lotters, S. & Jungfer, K. - H. & Schmidt, W. & Henkel, F. W. 2007: 513 |
| Grant, T. & Frost, D. R. & Caldwell, J. P. & Gagliardo, R. & Haddad, C. F. B. & Kok, P. J. R. & Means, D. B. & Noonan, B. P. & Schargel, W. E. & Wheeler, W. 2006: 171 |
Dendrobates biolat
| Brown, J. L. & Morales, V. & Summers, K. 2008: 5 |
| Medina-Muller, M. 2006: 1 |
| Roberts, J. L. & Brown, J. L. & von May, R. & Arizabal, W. & Presar, A. & Symula, R. & Schulte, R. & Summers, K. 2006: 381 |
| Christmann, S. P. 2004: 6 |
| Lotters, S. & Reichle, S. & Jungfer, K. - H. 2003: 1908 |
| Doan, T. M. & Arriaga, W. A. 2002: 108 |
| De La Riva, I. & Kohler, J. & Lotters, S. & Reichle, S. 2000: 3 |
| Schulte, R. 1999: 121 |
Dendrobates lamasi
| Roberts, J. L. & Brown, J. L. & von May, R. & Arizabal, W. & Presar, A. & Symula, R. & Schulte, R. & Summers, K. 2006: 381 |
| Christmann, S. P. 2004: 6 |
| Lotters, S. & Reichle, S. & Jungfer, K. - H. 2003: 1909 |
| Symula, R. & Schulte, R. & Summers, K. 2003: 453 |
| Schulte, R. 1999: 115 |
Dendrobates sirensis
| Schulte, R. 1999: 135 |
| Aichinger, M. 1991: 1 |
Dendrobates quinquevittatus
| Meede, U. 1980: 39 |