Ranitomeya imitator Schulte 1986
|
publication ID |
https://doi.org/10.11646/zootaxa.3083.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/1D338788-9503-151C-C8FC-9BA63C13FC80 |
|
treatment provided by |
Felipe (2021-08-23 20:40:41, last updated by Plazi 2023-11-04 13:58:37) |
|
scientific name |
Ranitomeya imitator Schulte 1986 |
| status |
|
Ranitomeya imitator Schulte 1986 View in CoL
Account authors: J.L. Brown, E. Twomey
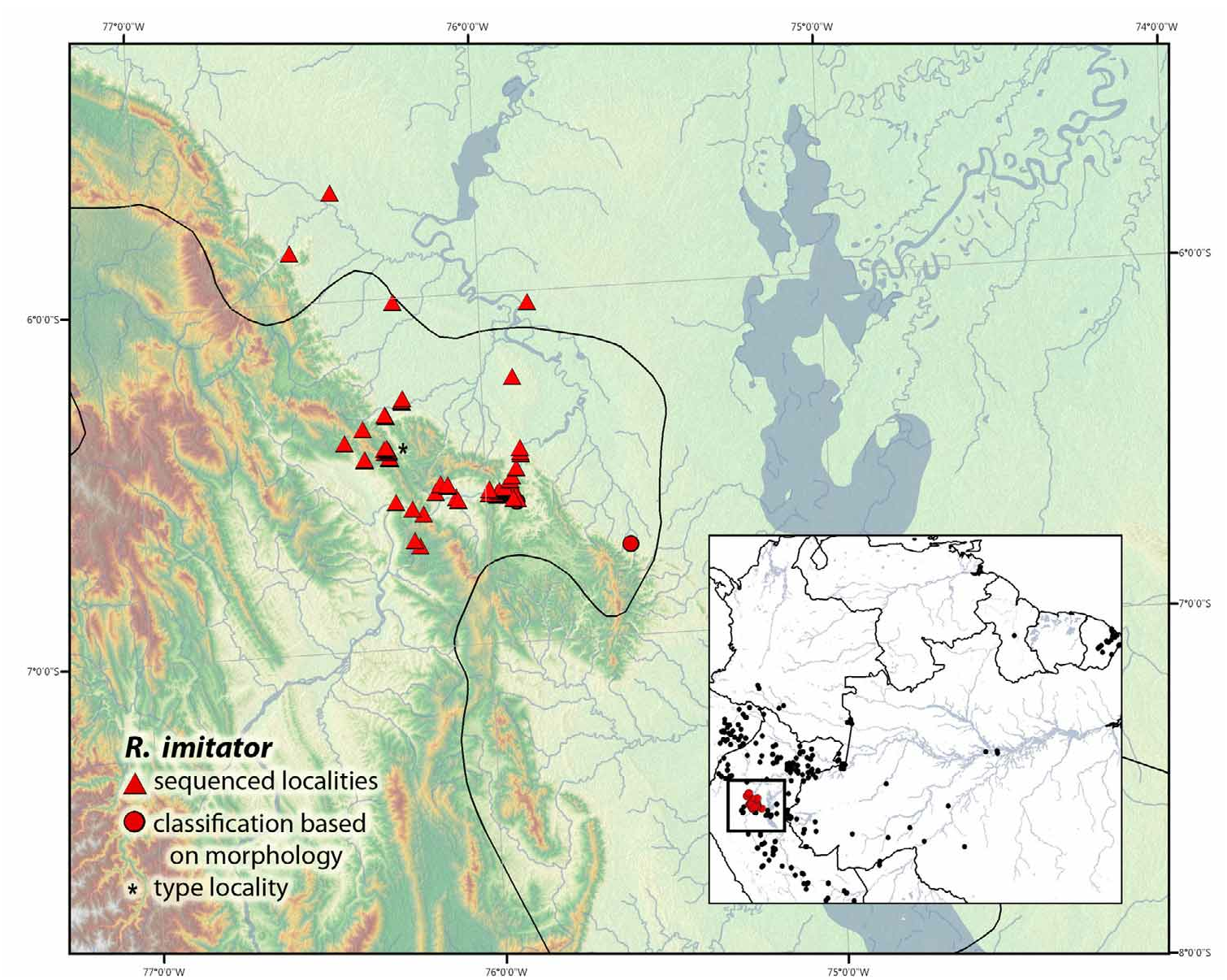
Figs. 3 View FIGURE 3 , 4 View FIGURE 4 , 9 View FIGURE 9 , 23 View FIGURE 23 (l–v), 24 (a–y), 25 (a–i), 28, 30
Tables 1, 4 – 6
Dendrobates quinquevittatus (non Steindachner 1864)—Silverstone 1975 (partim): p. 35; Zimmermann and Zimmermann 1984 (partim): p. 35; 1988 (partim): p. 132
Dendrobates ventrimaculatus View in CoL (non Shreve 1935)— Caldwell & Myers 1990 (partim): p. 18, Fig. 11 View FIGURE 11
Dendrobates imitator Schulte 1986: p. 11 View in CoL , Figs. 1–10 [MUSM BATR 10501 (holotype) collected by Rainer Schulte at km 33, near the village of San Jose, Carretera Tarapoto–Yurimaguas, San Martín, Peru];— Caldwell & Myers 1990: p. 17, Fig. 11c View FIGURE 11 ; Divossen 1999: p. 59; Symula et al. 2001: p. 2145, 2003: p. 452, Table 1, Figs. 1 – 6; Christmann 2004: p. 6, Figs. on p. 26, 27, 123 – 125; Brown et al. 2008a: p. 1140, Table 1, Fig. 1, 2008b: p. 1, Table 1, Figs. 1 – 3, 2009b: p. 478, Table 1 – 4, Fig. 3 View FIGURE 3 , 2009c: p. 148; Santos et al. 2009, by implication
Dendrobates imitator imitator View in CoL — Schulte 1999: p. 93 Figs. PB-015, PB-016; Lötters & Vences 2000: p. 253; Lötters et al. 2003: p. 1905; Christmann 2004: p. 142, Figs. on p. 142, 143
Dendrobates imitator intermedius Schulte 1999: p. 95 View in CoL , Figs. ZB-009, PB-037, PB-034, PB-036, PB-029, PB- 040, PB-031 [CRS BD 27 (holotype) collected by Rainer Schulte at “Huallaga Canyon, Department San Martín, Perú, 200 m NN.”];— Lötters & Vences 2000: p. 252; Christmann 2004: p. 30, Figs. on p. 30, 127, 128
Dendrobates imitator yurimaguensis Schulte 1999: p. 104 View in CoL , Figs. DB-056, PB-051, PB-035, PB-039 [CRS BD 41 (holotype) collected by Rainer Schulte on “Carretera Yurimaguas–Tarapoto, Alto Amazonas, Río Paranapura Drainage”];— Christmann 2004: p. 28, Figs. on p. 28, 29.
Ranitomeya imitator View in CoL — Bauer 1988: p.1; Grant et al. 2006: p. 171; Lötters et al. 2007: p. 478, Figs. 597–609; Brown et al. 2008c: p. 9; 2009a: p. 1877, Table 1, 2010: p. 436, Figs. 1, 2 View FIGURE 2 , 4 View FIGURE 4 , 5 View FIGURE 5 ; von May et al. 2008a: p. 396, Appendix 2
Ranitomeya intermedia View in CoL — Grant et al. 2006: p. 171; von May et al. 2008a: p. 396, Appendix 2
Dendrobates intermedius View in CoL — Santos et al. 2009, by implication
Background information. Few poison frogs have received as much attention from the public and the scientific community as R. imitator . Initial studies were focused on this species’ validity and its mimetic relationship with R. variabilis and R. summersi , each sensu this paper, e.g., Schulte (1986), Symula et al. (2001, 2003). Recently, this species has been the focus of several studies regarding the evolution of parental care, mate choice and the evolution of Müllerian mimicry (e.g., Brown et al. 2008ab, 2009b, 2010).
This species was discovered in the late 1980s by Rainer Schulte (1986). After naming the species in 1986, Schulte (1999) further subdivided it into three subspecies, R. imitator imitator , R. imitator yurimaguensis and R. imitator intermedius (described below as three color morphs). These names posed various taxonomic problems (see Lötters & Vences 2000) and immediately after their publication, the name imitator yurimaguensis was formally synonymized with the nominotypical form by Lötters & Vences (2000). However, imitator intermedius tentatively remained. Grant et al. (2006) elevated the status of imitator intermedius to full species (as R. intermedia ), but provided no justification for this taxonomic arrangement.
Ranitomeya imitator View in CoL has been the subject of several phylogenetic and population genetic studies and has been densely sampled throughout its known range ( Symula et al. 2001, 2003; E. Twomey & J.L. Brown, unpub. data). In addition, researchers have collected a considerable amount of acoustic, morphological and behavioral data on most populations of this species (E. Twomey, J.L. Brown & J. Yeager, unpub. data). None of the data from these studies indicate that R. imitator View in CoL is a species complex or adequately justifies the use of subspecies. In particular, recent population genetic studies have demonstrated that most color morphs are not reciprocally monophyletic, or even genetically distinct. Furthermore, within many populations different color morphs are known to coexist. To maintain the use of subspecies (and the resulting classification of R. intermedia View in CoL ) is misleading, ignoring considerable variation within and between most populations. Because of these reasons, we consider R. intermedia View in CoL to be a junior synonym of R. imitator View in CoL .
Definition and diagnosis. Assigned to the genus Ranitomeya due to the combination of the following characters: adult SVL <20.0 mm, dorsal coloration conspicuous, dorsolateral stripes, when present, extend to top of thighs, brightly colored throat, distinctive pale reticulation on limbs and venter (absent in some populations), dorsal skin smooth, finger I greatly reduced and shorter than finger II, finger discs II – IV greatly expanded, disc of finger two times wider than finger width, thenar tubercle conspicuous, toe discs III – V moderately expanded, toe webbing absent, larval vent tube dextral, adults use phytotelmata for reproduction and deposit eggs away from phytotelm, maxillary and premaxillary teeth absent. Vocal slits present in males.
Rantiomeya imitator is one of the most polymorphic poison frog species and a Müllerian mimic throughout most of its range, making identification of this taxon difficult. Furthermore, it possesses no single diagnostic morphological character that is consistent for all morphs. Three primary morphs exist: (i) Striped morph ( Fig. 24b–g, i– v View FIGURE 24 ), (ii) Spotted morph ( Fig. 23l–v View FIGURE 23 , 24a, h View FIGURE 24 ) and (iii) Banded morph ( Figs. 24w–y View FIGURE 24 , 25a–i View FIGURE 25 ). Although these three morphs predominate many populations do not exactly fit any of them (e.g., Fig. 24b, c, x, y View FIGURE 24 ). To thoroughly describe the morphs of a species that possesses such an immense variation undervalues complicated intrapopulation and interpopulation variation. Thus, the color morphs described below should be interpreted ‘loosely’ and in the context that they do not always represent a population or common ancestry.
(i) The Striped morph has a black dorsum with three thin yellow longitudinal stripes extending the length of the body. The presence of two paired spots on the nostrils creates the appearance of a yellow ‘cross’ anterior to the eyes (occasionally spots fuse to make a black “U,” Fig. 24o View FIGURE 24 ). Flanks are black and usually have a single yellow ventrolateral stripe that runs from the axilla to the groin. Legs, forelimbs and venter are black with fine light green to blue reticulation. The striped morph occurs throughout the lowlands northeast of the Cordillera Escalera, extending eastward, across the Río Huallaga , into the Pampas del Sacramento. It occurs as far north as Varadero (Department Loreto) but not reaching Río Marañón, and as far south as the northern Cordillera Azul, San Martín, Peru .
(ii) The Spotted morph has a dorsal ground color of orange, golden yellow, or green. The dorsum has small to medium black spots that occasionally fuse, resembling irregular stripes. This morph typically possesses paired black spots on the nostrils (occasionally these spots are fused and resemble a ‘U’). Legs, forelimbs and venter are the same as the Striped morph, but can also be finely to coarsely reticulate in the colors of teal to gold. This morph is montane and occurs throughout the Cordillera Escalera as far north as Balsapuerto, Loreto, Peru and as far south as Chazuta, San Martín, Peru.
(iii) The Banded morph is the most variable; however, the majority of individuals possess a black dorsum with symmetrical orange (occasionally yellow) bands (occasionally possessing a complete or partial vertebral stripe). This morph is found throughout the central Huallaga canyon, San Martín, Peru.
Ranitomeya imitator is a Müllerian mimic of R. summersi (banded morph) and R. variabilis (spotted and striped morphs, see discussion). Ranitomeya imitator can be distinguished from these species by its loud, trill-like call (compared to faint buzz-calls in the model species) which is audible from over 5 meters. It can be distinguished from R. variabilis by the presence of black paired nostril nose spots (single spot in R. variabilis ). Ranitomeya imitator can usually be distinguished from R. summersi by the presence of a black ovoid head spot (versus pentagonal in R. summersi ) and the absence of paired black gular spots (present in R. summersi ). This species, particularly the Striped morph, is also similar in morphology to some morphs of R. sirensis , although R. imitator lacks a yellow ventral patch and white axillary and inguinal spots typical of most forms of R. sirensis .
Tadpole. The description is based on a stage 26 tadpole from Cainarachi Valley, San José, San Martín, Peru. Body ovoid in dorsal view, wider near vent. Total length 17.5; body length 6.7; tail length 10.8, 62% of total length. Body width 3.7; body depth 2.2, 60% of body width. Eye well developed; naris small; distance from naris to anterior edge of eye 1.6. Eye positioned dorsally on head, directed dorsolaterally. Spiracle well developed; vent tube dextral.
Tip of tail bluntly rounded. Tail muscle height at base of tail 1.7; tail muscle width at base of tail 0.9; maximum tail height 1.9. Dorsal fin slightly higher than ventral fin.
Oral disc ventral, weakly emarginate; transverse width 1.4, 38% of body width. Single row of small papillae present laterally and ventrally; dorsal gap where papillae absent. LTRF 2(2)/3(1) with A-1 developed on upper labium, A-2 with wide medial gap (one third total width of A-2); P-1 on lower labium with narrow medial gap; P- 3, 55% width P-1; P-2 equal in width P-1.
Color in preservative. Head whitish, mouthparts visible from above. Whitish abdomen, mostly transparent, intestinal coils black. Tail musculature uniform tan, dorsal and ventral fins opaque white.
Natural history. This species is a Müllerian mimic of several congeneric species throughout its range (see discussion). Ranitomeya imitator exhibits a seasonal monogamous mating system and biparental care. After one to two embryos hatch, the male carries the tadpoles on its back and deposits each individually in a phytotelm. Both parents then return weekly and the female deposits trophic eggs after being stimulated by the male. The tadpoles are continually fed until metamorphosis. For details and discussion of these behaviors and their evolution, and the natural history of this species see Brown et al. (2008a, b, 2009b, 2010).
Vocalizations. The call of R. imitator is stereotypical for members of the vanzolinii group. It is a loud trill, notes 0.44 – 1.07 sec in length, repeated at 7 – 11 notes per minute. Despite major morphological differences between populations, acoustic divergence between populations appears to be minimal (E. Twomey, unpub. data).
Distribution. This species occurs within Amazonian rainforests of Peru (Departments: Loreto and San Martín), Fig. 30 View FIGURE 30 .
Conservation status. Following the IUCN Red List categories and criteria ( IUCN 2010), we tentatively suggest listing this species as Least Concern (LC). Its distribution is estimated to be around 10,000 km 2. Because of the pet market demand and extreme morphological variation over a small geographic area, some color morphs maybe vulnerable.
Bauer, L. (1988) Pijlgifkikkers en verwanten: de familie Dendrobatidae. Het Paludarium, 1 Nov, 1988, 1 - 6.
Brown, J. L., Morales, V. & Summers, K. (2008 a) Divergence in parental care, habitat selection and larval life history between two species of Peruvian poison frogs: an experimental analysis. Journal of Evolutionary Biology, 21, 1534 - 1543.
Brown, J. L., Twomey, E., Pepper, M. & Rodriguez, M. S. (2008 c) Revision of the Ranitomeya fantastica species complex with description of two new species from Central Peru (Anura: Dendrobatidae). Zootaxa, 1823, 1 - 24.
Caldwell, J. P. & Myers, C. W. (1990) A new poison frog from Amazonian Brazil, with further revision of the quinquevittatus group of Dendrobates American Museum Novitates, 2988, 1 - 21.
Christmann, S. P. (2004) Dendrobatidae - Poison Frogs - A Fantastic Journey through Ecuador, Peru and Colombia (Volumes I, II & III).
Divossen, H. (1999) Dendrobates fantasticus in the field and in the terrarium. Aquarium (Bornheim), 355, 58 - 60.
Grant, T., Frost, D. R., Caldwell, J. P., Gagliardo, R., Haddad, C. F. B., Kok, P. J. R., Means, D. B., Noonan, B. P., Schargel, W. E. & Wheeler, W. (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia, Athesphatanura, Dendrobatidae). Bulletin of the American Museum of Natural History, 299, 1 - 262.,
IUCN (2010) IUCN Red List Categories and Criteria: Version 8.1. IUCN Species Survival Commission, IUCN, Gland, Switzerland and Cambridge, UK, 85 pp.
Lotters, S. & Vences, M. (2000) Bemerkungen zur Nomenklatur und Taxonomie peruanischer Pfeilgiftfrosche (Anura: Dendrobatidae: Dendrobates, Epipedobates). Salamandra, 36, 247 - 260.
Lotters, S., Reichle, S. & Jungfer, K. - H. (2003) Advertisement calls of neotropical poison frogs (Amphibia: Dendrobatidae) of the genera Colostethus, Dendrobates and Epipedobates, with notes on dendrobatid call classification. Journal of Natural History, 37, 1899 - 1911,
Lotters, S., Jungfer, K. - H., Schmidt, W. & Henkel, F. W. (2007) Poison Frogs: Biology, Species and Captive Husbandry Edition Chimaira, Frankfurt am Main, 668 pp.
Santos, J. C., Coloma, L. A., Summers, K., Caldwell, J. P., Ree, R. & Cannatella, D. C. (2009) Amazonian Amphibian Diversity Is Primarily Derived from Late Miocene Andean Lineages. PLoS Biol, 7, e 1000056.
Schulte, R. (1986) Eine neue Dendrobates - Art aus Ostperu (Amphibia: Salentia: Dendrobatidae). Sauria, 8, 11 - 20.
Schulte, R. (1999) Pfeilgiftfrosche Artenteil - Peru . INBICO, Wailblingen, Germany, 294 pp.
Shreve, B. (1935) On a new Teiid and Amphibia from Panama, Ecuador, and Paraguay. Occasional Papers of the Boston Society of Natural History, 8, 209 - 218.
Symula, R., Schulte, R. & Summers, K. (2001) Molecular phylogenetic evidence for a mimetic radiation in Peruvian poison frogs supports a Mullerian mimicry hypothesis. Proccedings of the Royal Sociey of Lond Series B-Biological Science, 268, 2415 - 2421.
Symula, R., Schulte, R. & Summers, K. (2003) Molecular systematics and phylogeography of Amazonian poison frogs of the genus Dendrobates. Molecular Phylogenetics and Evolution, 26, 452 - 75.
von May, R., Catenazzi, A., Angulo, A., Brown, J. L., Carrillo, J., Chavez, G., Cordova, J. H., Curo, A., Delgado, A., Enciso, M. A., Gutierrez, R., Lehr, E., Martinez, J. L., Medina-Muller, M., Miranda, A., Neira, D. R., Ochoa, J. A., Quiroz, A. J., Rodriguez, D. A., Rodriguez, L. O., Salas, A. W., Seimon, T., Seimon, A., Siu-Ting, K., Suarez, J., Torres, J. & Twomey, E. (2008 a) Current state of conservation knowledge of threatened amphibian species in Peru. Tropical Conservation Science, 1, 376 - 396.
Zimmermann, H. & Zimmermann, E. (1984) Durch Nachzucht erhalten: Baumsteigerfrosche. Dendrobates quinquevittatus und D. reticulatus. Aquarien Magazin, 18, 35 - 41.
FIGURE 2. Illustrated guide to morphological terminology. A. Finger and hand morphology: i. Finger I (far left) <Finger II, thenar tubercle (= inner metacarpal tubercle) present (depicted by arrow), and greatly expanded finger discs in Fingers II-IV. Inset depicts Finger I and a thenar tubercle which is clearly visible. Note that in some Ranitomeya this is trait reduced and difficult to view (as in main picture) (Ranitomeya variabilis pictured, inset of R. benedicta). ii. Finger I ≈ Finger II, thenar tubercle absent. (Adelphobates quinquevittatus pictured) iii. Weakly expanded finger discs in Fingers II-IV (Excidobates captivus pictured). B. Stripes: i. Middorsal (follows vertebral column), dorsolateral (extends from eye to either upper thigh, as pictured, or to vent), ventrolateral (running from groin to axilla) and labial stripe (stripe that extends from shoulder around upper lip)(R. sirensis pictured). ii.. Oblique lateral stripe (extends from groin to eye, as in picture stripe is incomplete anteriorly). Unlabeled arrow depicts a dorsolateral stripe that does not reach thigh, a characteristic of certain species of Andinobates (type ‘A’ in Grant et al. 2006). (Andinobates claudiae pictured). C. Limb patterns: i. Distinct limb reticulation/spotting (characteristic of most species of Ranitomeya) (R. variabilis pictured). ii. Wavy stripes (not classified as distinct limb reticulation) (R. summersi pictured). iii. Patternless. Typical of most Andinobates species (R. sirensis pictured). D. Diagnostic head patterns: i. Large black “oval” on head (R. imitator pictured). ii. Large black “pentagon” or “five-point star” on head (R. summersi pictured). iii. Black band across head entirely covering eyes (known only in a single population of this species near the Pongo de Manseriche, Peru) (R. fantastica pictured). E. Nose spots. i. Two nose spots (R. imitator pictured). ii. Single nose spot. (R. variabilis pictured). iii. Frontward-turned “U” on the tip of snout. (R. toraro pictured). F. Geographical distribution. West: distribution within Andes, west of Andes, or in Central America. East: distribution east of Andes (including Guiana Shield) or in east-Andean versant. G. Dorsal patterns: i.“Y-shape”. Space between stripes create black pattern which forms a black Y on the back. (R. variabilis pictured). ii. Merging of the obliquelateral and dorsolateral stripes (R. variabilis pictured). iii. Broken dorsolateral stripes (R. flavovittata pictured). iv. Spotting (R. imitator pictured). H. Key ventral characters: i. Distinctive throat coloration and ventral reticulation (also shown in H-ii & H-iii) (R. reticulata pictured). ii. Belly patch (R. sirensis pictured). iii. Gular spots (single or paired dark spots at corner of mouth) (R. amazonica pictured). iv. Marbled pattern (not classified as reticulation) (Andinobates virolinensis pictured).
FIGURE 3. A consensus Bayesian phylogeny based on 1011 base pairs of aligned mitochondrial DNA sequences of the 12S (12s rRNA), 16S (16s rRNA) and cytb (cytochrome-b gene) regions. Thickened branches represent nodes with posterior probabilities 90 and greater, other values are shown on nodes. Taxon labels depict current specific epithet, number in tree, the epithet being used prior to this revision (contained in parentheses), and the collection locality. A. Top segment. B. Middle segment. C. Bottom segment of phylogeny.
FIGURE 4. Putative species tree for Andinobates, Excidobates, and Ranitomeya. Placement of species where molecular data were lacking (A. altobueyensis, A. viridis, A. abditus, A. daleswansoni and R. opisthomelas) was based on morphology. Andinobates altobueyensis and A. viridis were placed as sister taxa due to the absence of dark pigmentation on dorsal body and limbs and overall similar dorsal coloration and patterning. These species were placed as sister to A. fulguritus (sequenced) on the basis of similar dorsal coloration (bright green to greenish-yellow). Andinobates opisthomelas was placed in the bombetes group in a polytomy with A. bombetes and A. virolinensis (both sequenced) due to their similar advertisement calls and morphology, particularly their red dorsal pattern and marbled venter. Andinobates daleswansoni was placed as sister to A. dorisswansonae due to the absence of a well-defined first toe in both species. Andinobates abditus was placed in the bombetes group based on a larval synapomorphy which appears to be diagnostic of that group (wide medial gap in the papillae on the posterior labium). However, A. abditus was placed as the sister species to all other members of the bombetes group due to the absence of bright dorsal coloration and isolated geographic distribution. Andinobates abditus is currently the only species of its genus known to occur in the east-Andean versant, thus its placement remains speculative until molecular data become available. Photo credits: Thomas Ostrowski, Karl-Heinz Jungfer, Victor Luna-Mora, Giovanni Chaves-Portilla.
FIGURE 5. Andinobates Plate 1. minutus group: A–G: Andinobates claudiae and habitat (all from Bocas del Toro, Panama. Photos T. Ostrowski); A & B: Buena Esperanza; C–F: Isla Colon; G: Cerro Brujo; H: tadpole in phytotelm; I: habitat in Bocas del Toro, Panama. J–M: Andinobates minutus (all from Colombia. Photos DMV unless noted): J & K: Buenaventura, Valle del Cauca; L: Quibdó, Chocó; M: Baudó, Chocó (photo J. Mejía-Vargas). fulguritus group: N–V: Andinobates fulguritus (all from Colombia, photos DMV unless noted): N: Baudó, Chocó (photo J. Mejía-Vargas); O: Playa de Oro, Chocó (type locality); P–R: Uraba, Chocó. S–V: Anchicayá, Valle del Cauca. (nΦ = number of individual in phylogeny, Ω = population sampled in phylogeny).
FIGURE 9. Known elevation distributions of Ranitomeya. Dotted line is mean for all samples. Dark boxes display the total elevation range of each species, within each contains a corresponding box plot.
FIGURE 11. Ranitomeya Plate 2. defleri group: A–H: Ranitomeya toraro (all from Brazil); A-B: Rio Branco, Acre (T. Grant); C-F: Upper Jurua, Acre (unknown). From Colombia: G: Leticia, Amazonas (Jose Manuel Padial, Ω); H: Axil of Aechmea sp. with two R. toraro embryos, near Boca do Acre, Amazonas, BZ (MBS). I: Adelphobates quinquevittatus, near Boca do Acre, Amazonas, BZ (PRMS); J: R. uakarii near Porto Walter, Acre, BZ (JPC, Ω); K: Tadpole of R. toraro (MBS). (Ω = population sampled in phylogeny).
FIGURE 23. Ranitomeya Plate 6. vanzolinii group: A & B: Ranitomeya cyanovittata: Sierra del Divisor, Ucayali, Peru (G. Knell and D. Vasquez, 1:Ω,2: 1Φ). C & D: Ranitomeya yavaricola (all from Loreto, Peru): C: Rio Blanco (G. Knell); D: Lago Preto (PPP, Ω). E– I: Ranitomeya flavovittata (all from Quebrada Blanco, Loreto, Peru (Photo credits: JLB, ET and PPP, Ω). J–K: Ranitomeya vanzolinii Atalaya, Ucayali, Peru (J. Yeager). L–V: Ranitomeya imitator (All from San Martin, Peru): L–O: Upper Canarachi Valley (‡); P– Q: Tarapoto (‡); R: Shapaja (‡); S: Chumia (‡) and T–V: Chazuta (Ω). (nΦ = number of individual in phylogeny, Ω = population sampled in phylogeny, ‡ = genetically sampled, but not included in our phylogeny).
FIGURE 24. Ranitomeya Plate 7. vanzolinii group: A–Y: Ranitomeya imitator (all from San Martin, Peru unless noted): A-B: Chazuta (Ω); C: Central Huallaga Canyon (‡); D-H: Callanayacu (‡); I-J: Lower Huallaga Canyon (‡); K-Q: Pongo de Cainarachi (Ω); R- S: Balsapuerto, Loreto (‡); T–V: Varadero, Loreto (‡) and W–Y: Curiyacu (‡). (Ω = population sampled in phylogeny, ‡ = genetically sampled, but not included in our phylogeny).
FIGURE 25. Ranitomeya Plate 8. vanzolinii group: A–I: Ranitomeya imitator Curiyacu, San Martin, Peru (‡). J–T: Ranitomeya sirensis (all from Peru unless noted): J-L: CICRA Station, Madre de Dios (Rio Los Amigos, Ω); M: near Rio Branco, Acre, Brazil (PRMS); N & O: Central Rio Urubamba, Cusco (G. Chavez); P & Q: Tingo Maria, Huánuco (ET, Ω); R: Bamboo forest, R. sirensis often uses the phytotelmata within bamboo for tadpole deposition, Tingo Maria, Huánuco (ET); S: Aguaytía, Ucayali; T: Codo del Pozuzo, Huánuco (20Φ). (nΦ = number of individual in phylogeny, Ω = population sampled in phylogeny, ‡ = genetically sampled, but not included in our phylogeny).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ranitomeya imitator Schulte 1986
| Brown, Jason L., Twomey, Evan, Amézquita, Adolfo, Souza, Moisés Barbosa De, Caldwell, Jana- Lee P., Lötters, Stefan, May, Rudolf Von, Melo-Sampaio, Paulo Roberto, Mejía-Vargas, Daniel, Perez-Peña, Pedro, Pepper, Mark, Poelman, Erik H., Sanchez-Rodriguez, Manuel & Summers, Kyle 2011 |
Ranitomeya intermedia
| von May, R. & Catenazzi, A. & Angulo, A. & Brown, J. L. & Carrillo, J. & Chavez, G. & Cordova, J. H. & Curo, A. & Delgado, A. & Enciso, M. A. & Gutierrez, R. & Lehr, E. & Martinez, J. L. & Medina-Muller, M. & Miranda, A. & Neira, D. R. & Ochoa, J. A. & Quiroz, A. J. & Rodriguez, D. A. & Rodriguez, L. O. & Salas, A. W. & Seimon, T. & Seimon, A. & Siu-Ting, K. & Suarez, J. & Torres, J. & Twomey, E. 2008: 396 |
| Grant, T. & Frost, D. R. & Caldwell, J. P. & Gagliardo, R. & Haddad, C. F. B. & Kok, P. J. R. & Means, D. B. & Noonan, B. P. & Schargel, W. E. & Wheeler, W. 2006: 171 |
Dendrobates imitator imitator
| Christmann, S. P. 2004: 142 |
| Lotters, S. & Reichle, S. & Jungfer, K. - H. 2003: 1905 |
| Lotters, S. & Vences, M. 2000: 253 |
| Schulte, R. 1999: 93 |
Dendrobates imitator intermedius
| Christmann, S. P. 2004: 30 |
| Lotters, S. & Vences, M. 2000: 252 |
| Schulte, R. 1999: 95 |
Dendrobates imitator yurimaguensis
| Christmann, S. P. 2004: 28 |
| Schulte, R. 1999: 104 |
Ranitomeya imitator
| Brown, J. L. & Twomey, E. & Pepper, M. & Rodriguez, M. S. 2008: 9 |
| von May, R. & Catenazzi, A. & Angulo, A. & Brown, J. L. & Carrillo, J. & Chavez, G. & Cordova, J. H. & Curo, A. & Delgado, A. & Enciso, M. A. & Gutierrez, R. & Lehr, E. & Martinez, J. L. & Medina-Muller, M. & Miranda, A. & Neira, D. R. & Ochoa, J. A. & Quiroz, A. J. & Rodriguez, D. A. & Rodriguez, L. O. & Salas, A. W. & Seimon, T. & Seimon, A. & Siu-Ting, K. & Suarez, J. & Torres, J. & Twomey, E. 2008: 396 |
| Lotters, S. & Jungfer, K. - H. & Schmidt, W. & Henkel, F. W. 2007: 478 |
| Grant, T. & Frost, D. R. & Caldwell, J. P. & Gagliardo, R. & Haddad, C. F. B. & Kok, P. J. R. & Means, D. B. & Noonan, B. P. & Schargel, W. E. & Wheeler, W. 2006: 171 |
| Bauer, L. 1988: 1 |
Dendrobates imitator
| Brown, J. L. & Morales, V. & Summers, K. 2008: 1140 |
| Christmann, S. P. 2004: 6 |
| Symula, R. & Schulte, R. & Summers, K. 2001: 2145 |
| Divossen, H. 1999: 59 |
| Caldwell, J. P. & Myers, C. W. 1990: 17 |
| Schulte, R. 1986: 11 |