Salea horsfieldii Gray, 1845
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4563.3.9 |
|
publication LSID |
lsid:zoobank.org:pub:16A92AEA-99BD-415A-8F4A-A9A73DEDFCA |
|
DOI |
https://doi.org/10.5281/zenodo.5943008 |
|
persistent identifier |
https://treatment.plazi.org/id/194AA70F-FFDD-B451-38D8-FB3CAD33FBDC |
|
treatment provided by |
Plazi |
|
scientific name |
Salea horsfieldii Gray |
| status |
|
Redescription of Salea horsfieldii Gray
Gray (1845) based his description of this species on two syntypes, both of which were females. He stated that this species is found in ‘ India, Afghanistan’. Günther (1864), however, reported that the evidently wrong attribution of those specimens’ origin to Afghanistan was actually caused by a mistake on the jar label and only ‘India’ was thereafter considered as the type locality ( Boulenger 1885; Smith 1935). Both syntypes are in good state of preservation. Here we redescribe them in detail. The characters of NHM 1946.8.14.12 appear first and those of NHM 1946.8.14.11 are mentioned in square brackets where characters differ. Morphometric and meristic data are shown in Table 2.
Redescription of the syntypes (based on NHM 1946.8 About NHM .14.12 and NHM 1946.8.14.11): ( Table 2, Figure 2 View FIGURE 2 ) Adult females, SVL 79 mm [75 mm]; tail measuring twice as long as the SVL (TL/ SVL 1.94 [2.06]; TrL 37.4 mm [33.4 mm]; although the body was probably compressed in life, after almost 172 years of preservation the trunk has become somewhat flattened (BW/BH 1.23 [1.1]); head moderately sized (HL/SVL 0.25 [0.27]), elongated (HW/ HL 0.71 [0.59]) and almost as wide as high (HW/HH 1.01 [1.13]); eye moderate sized (ED/HL 0.24); orbit diameter is greater than tympanum diameter; EErD nearly equal to one ED; END subequal to ED whereas ESD greater than ED.
Snout obtusely pointed; rostral scale nearly thrice wider than high; rostral separate from nasal by one scale; scales on snout rugose, not smaller than those on occiput; keeled scales on supraocular region smaller than those on frontal and occipital region; scales on frontal and occipital region rugose and those on the midline of these two regions are somewhat elongated; no post-orbital spines; canthus rostralis and supracilliary edge sharp; CN (R/L) 3/ 3 [2/3]; nasal single and hexagonal with oval nostril and nasal touches 1 st and 2 nd SL; SUB (R/L) 8/8 (8/10); no ‘wedge’ scales between SUB and SL; SL (R/L) 8/8 [8/8], SL squarish except the 1 st and the last one which are wedge-shaped; tympanum exposed; 3 large, rugose scales from behind orbit to above tympanum; no supratympanic spines; a very low nuchal crest, formed of two rows of strongly keeled, compressed scale rows and the nuchal crest resembles a serrated ridge without well defined spines; mental subpentagonal with two small postmentals behind it; IL (R/L) 7/7; gular scales strongly keeled, imbricate and slightly mucronate; no distinct gular sac apart from an indistinct fold; no transverse fold across gular region;
Ante-humeral fold absent; dorsal scales keeled, imbricate and directed backward (scales on ventro-lateral part of trunk, however, show a slight downward tilt); homogenous dorsal scales are mostly subequal with very lighter coloured ones on flank which are a little larger than adjacent scales; SM 36 [38]; no dorsal crest; ventrals strongly keeled, imbricate and mucronate; VEN 53 [50].
Forearm subequal to upper arm in length (FAL/UAL 1.11 [1.25]); Femur subequal to crus in length (FL/CL 1.05 [0.93]); scales on limbs rhomboid, keeled; five slender digits with recurved claws on both manus and pes; lamellae under digits bicarinate; FnLam (R/L) 17/18 [23/21] and TLam (R/L) 24/21 [24/23].
Tail slightly compressed with rhomboid, keeled scales.
Top of head of NHM 1946.8.14.12 a light brown colour with dark brown scales scattered randomly and several scales have dark brown edges; loreal, nasal and supralabial region whitish cream with brown edging on posterior border of SL; a dark brown post-orbital streak which ends at the angle of jaw; lower jaw cream, some gular scales with dark brown borders at their edge; in this specimen dorsum is light brown with five dark brown blotches on dorsal midline; flanks blotched and mottled with dark brown; limbs alternatively banded with light and dark brown with a light coloured streak behind thigh; venter whitish cream; tail dark and light banded. Overall ground colouration of dorsum NHM 1946.8.14.11 is grayish brown; patterns of head and limbs similar to the former specimen; dorsal pattern is very faint in this specimen; venter cream.
Variations: Ranges of mensural and meristic characters are summarized in Table 2. This species shows sexual dimorphism in several characters and one of them—nuchal and dorsal ornamentation of males—has recently been shown to be present from a very young, even juvenile age ( Daniel et al. 2017). Males have a prominent nuchal crest composed of flattened lanceolate spines longest of which are about one and half times of orbit diameter. Dorsal crest of males, which is separated from nuchal crest by 3–5 scales, consists of 16–20 spines from beginning point to sacrum. The dorsal crest continues over the anterior part of tail as a much lower crest. Nuchal and dorsal spines are composed of single, elongated, lanceolate scales with one upwardly directed, keeled scale on each side of their base. Nuchal and dorsal spines are banded alternatively with black and white. Males have a small gular sac. Trunk and tail of males are more strongly compressed than they are in females. Females also have a low nuchal crest composed of two rows of compressed, keeled scales (5/ 6 in number). Females do not possess dorsal crest although adult females usually have a trace of a serrated ridge of keeled scales along dorsal midline.
Dorsal, ventral and gular scales are slightly more overlapping in males than they are in females.
Colouration of this species is highly variable. Most of the specimens fade to a light brown colour in preservative over time, but the specimens we observed in nature have an olivaceous green background colour. Most of the preserved specimens that we observed and those we observed in the wild had two cream dorsolateral stripes. Some specimens (e.g. ZSI 26220), however, lacked these stripes. Some males (e.g. NHM 1867.8.11.13) show a striking colour pattern where the ground colour is yellowish cream with a dark blackish brown border along the edges of their dorsal scales and also a mottling of the same colour. The dorsal surface of the males’ head sometimes have a dark brown ground colour with an orange yellow spot at the centre of each scale. Generally dark brown transverse blotches are present on the dorsal surface of all specimens and these are interrupted by the light dorsolateral stripes in the specimens which possess these. In several specimens the post-orbital stripe, instead of reaching the angle of mouth, runs along the sides of nape up to forelimb. Light coloured (often white in males), larger sized scales are present sporadically on flank.
In one specimen we collected from Pykara (Tamil Nadu, India)—ZSI 26219—there are 5 ‘wedge’ scales present on the right side of head and 6 on the left side ( Figure 3 View FIGURE 3 ). These are truly ‘wedge’ scales in the sense that these are located in between 4 scales—2 SUB and 2 SL—and therefore cannot be regarded as mere fragmentation of any SUB or SL. However, one specimen (CAS 94351) from a place near Pykara in the collection of the California Academy of Science (California) did not possess such scales whereas one of the two syntypes of Salea jerdonii (NHM 1946.8.14.4) did have ‘wedge’ scales; these were, however, much smaller in size. The ‘wedge’ scales of the specimen from Pykara can therefore be considered an example of intrapopulational variation.
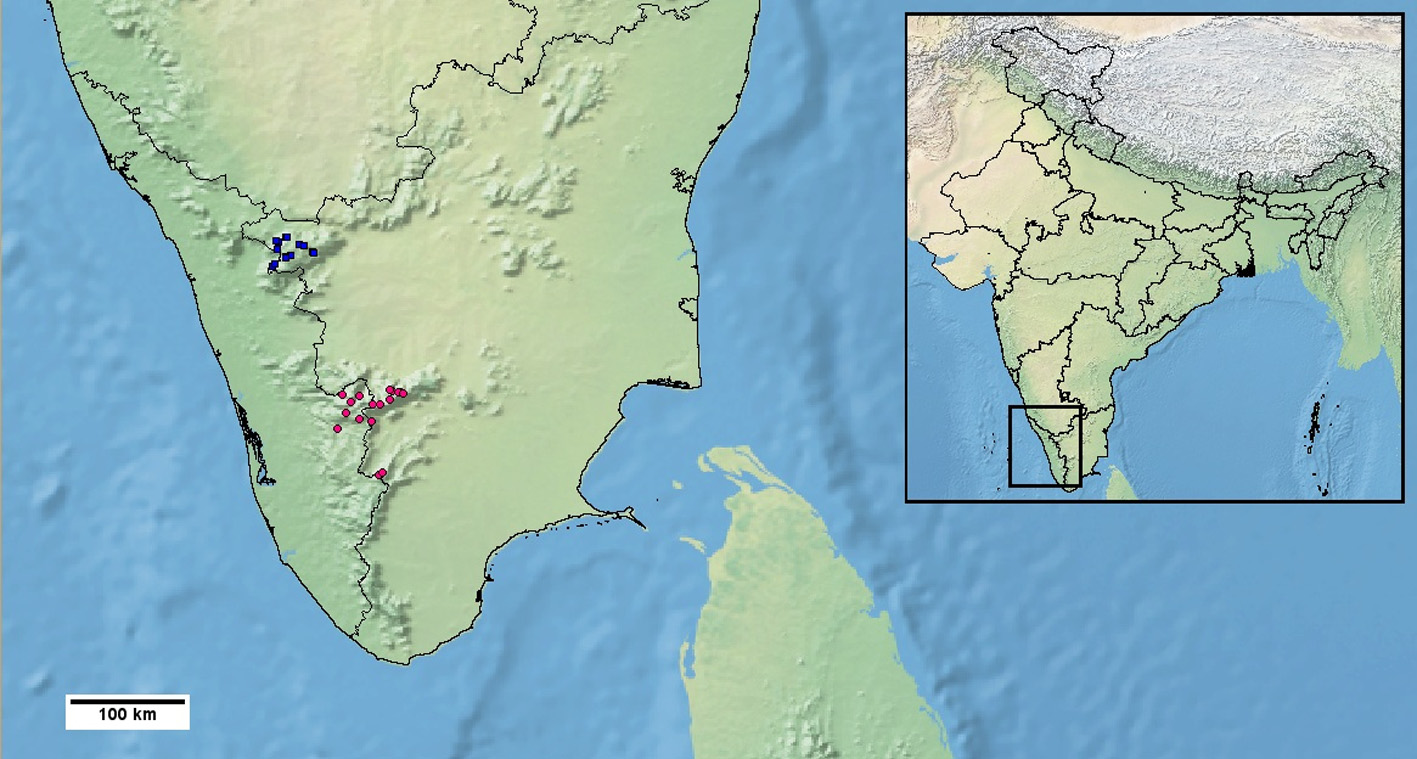
Distribution: Salea horsfieldii has been recorded only from the Nilgiri hill range situated north of the Palghat gap, in western Tamil Nadu state ( Figure 7 View FIGURE 7 ). This record is undisputed. Roux (1928) mentioned one specimen from Vandaravu hill which is situated south of Palghat gap. Probably following Roux’s work, Smith (1935) included both Nilgiri and Palni hills in its range. Several authors (e.g. Murthy 1985; Daniel 2002; Das 2002) have subsequently included Palni hills in the distributional range of this species. However, Bhupathy & Kannan (1997) stated that during their study on the agamids of Western Ghats they failed to find specimen of this species anywhere south of the Palghat gap. Srinivas et al. (2008) agreed with the viewpoint of the aforementioned authors regarding the distribution of S. horsfieldii . Ganesh & Aengals (2011) reported on a specimen of S. horsfieldii collected by T. S. N. Murthy of Zoological Survey of India. It reportedly originated from Thandikudi in the Palni hills. The rather brief description of the specimen and associated photograph confirms the identification as S. horsfieldii according to current taxonomy. However, any of the aforesaid dubious recorded localities is most likely to be the result of collections becoming muddled. The Palghat gap is a very important biogeographic barrier and deeply divergent sister clades of several animal groups are often found on opposite sides of this barrier (e.g. John et al. 2013; Robin et al. 2015; Vijayakumar et al. 2016; Robin et al. 2017). To the best of our knowledge, no recent publications mention any first-hand account of sightings of this species in the Palni hills. The third author of the present paper did not come across it in the Palni hills during field work in 2011. Very recently Daniel et al. (2017) supported Bhupathy & Kannan’s (1997) opinion on the distribution of S. horsfieldii and regarded it to be a Nilgiri hills endemic. For all these reasons, we also regard the record of this agamid from Palni hills as dubious and in need of review. We would like to mention here, given that the Palghat gap is a biogeographic barrier, there is strong possibility that should this population be found in the Palni hills, they may be genetically divergent and therefore a different evolutionary lineage. One record from Kudremukh hills is generally considered to be erroneous ( Ganesh & Aengals 2011; Srinivasulu et al. 2014). Kelaart (1854) listed S. jerdonii , a synonym of S. horsfieldii , for Sri Lanka but that has proved to be in error ( Srinivasulu et al. 2014).
Natural History: This species is an inhabitant of humid montane ‘shola’ forests and tea plantations situated between 1600–2500 meters above sea level ( Das 2002; Srinivasulu et al. 2014). Smith wrote that this lizard frequents ‘bushes, hedges, and gardens’. We observed them in the morning hours (0900 hr) through to noon (1400 hr) on both cloudy and sunny days. All the members of this species were seen by us in either ‘shola’ type forests or tea estates at 1.5–10 feet above the ground on bushes or trees. The overall gait of this lizard is like that of Calotes spp. but it is considerably slower in movement than the latter. Wall (1922) stated that males of this species assume a bright green colour with yellow head and gular sac when excited. We observed one excited female developing a bluish colour along the edges of some of its dorsal scales. This species primarily eats insects ( Das 2002) but recently Kumar et al. (2017) recorded a case of predation on Raorchestes signatus , an endemic bush frog species, by this agamid lizard. Interestingly, we too often found Raorchestes spp. in the same locality where S. horsfieldii was observed, giving further evidence of their predator-prey interaction. Platyplectrurus madurensis and Psammophilus dorsalis were observed to live sympatrically with S. horsfieldii during our survey. They lay 3– 4 eggs (17 X 9 mm) ( Smith 1935) and females with eggs were seen in September ( Bhupathy & Kannan 1997).
| NHM |
University of Nottingham |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.