Bothrioplana sinensis Wang & He
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4179.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:7CA48761-3F3D-404F-B8D8-476F0EB06293 |
|
DOI |
https://doi.org/10.5281/zenodo.5674327 |
|
persistent identifier |
https://treatment.plazi.org/id/192187CA-FFBB-FFAC-5AAA-1ABEFCD9FBDF |
|
treatment provided by |
Plazi |
|
scientific name |
Bothrioplana sinensis Wang & He |
| status |
sp. nov. |
Bothrioplana sinensis Wang & He , n. sp.
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4. A View FIGURE 5. A )
Type material. Holotype: PLA-B001, mounted specimens. Paratype: PLA-B002– PLA-B006 (PLA-B002–PLA- B003, mounted; PLA-B004–PLA-B006, serial section).
Collection locality. In an artificial lake of Shenzhen University campus, Shenzhen, Guangdong, China (22°31'44.81"N, 113°55'52.17"E). GoogleMaps
Etymology. The species is named because it was discovered in China.
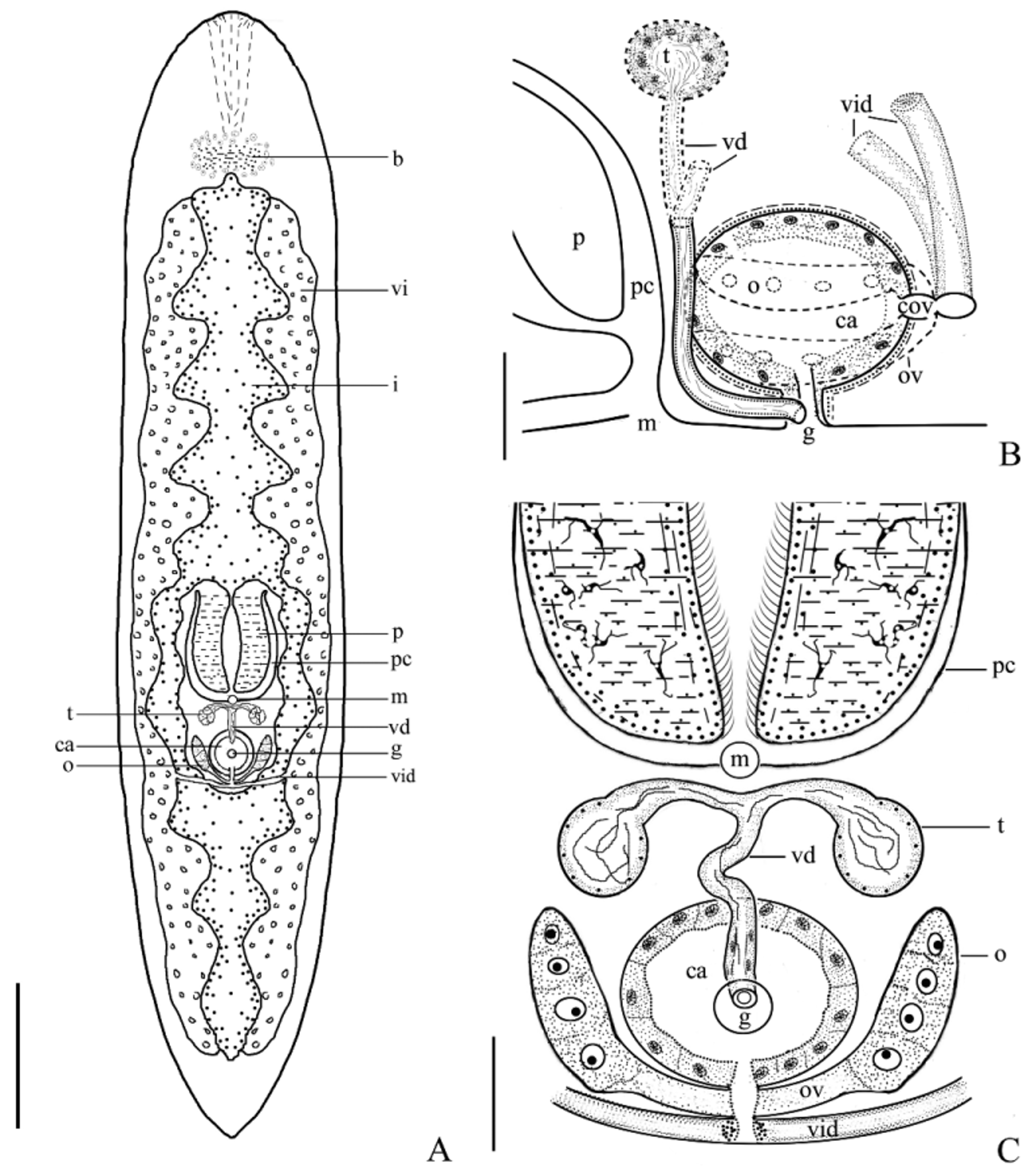
Description. The mature individual is 3–5 mm in length, transparent, flat and appears in an elongated shape. The body surface has no pigment. The width of the middle part of the body is 0.7–1.0 mm ( Fig. 1 View FIGURE 1 A, Fig. 3 View FIGURE 3 A). The anterior end is in a circular arc shape, having no eyespot or statocyst. The tail is in a “V” shape.
Digestive system. The light brown digestive tract in the median line of the body is divided into two at pharynx, which then integrate into one at the posterior end of the copulatory organs. The caecus at both sides of intestinal canal are relatively long in the anterior part of the body, while relatively short in the posterior part ( Figs. 1 View FIGURE 1 A, 3A). The mouth (m) is in the middle part of the body. The transformable plicate pharynx (p) is 205–505 µm in length, lying in the anterior part of the mouth. The vitellaria (vi) are distributed in both sides of intestinal canals. The butterfly-shaped brain (b) at the anterior 1/10 position of the body is composed of peripheral neuroglia cell and interior collagenous fiber ( Figs. 1 View FIGURE 1 D–E).
Reproductive system. Hermaphrodite with a gonopore (g). The reproductive organ is located posterior to the pharynx (p) ( Figs. 1 View FIGURE 1 F–G, 2D–G, 3A–C). A pair of spherical-shaped testes (t) is located dorsally behind the pharynx. The testes, 27–37 µm in diameter, contain different development stages of spermatids. At the late stage of testis development, the spermatid structure is not obvious, but the vasa deferentia (vd) and the testes are filled with mature sperm (s). The vasa deferentia originate from the internal sides of the two testes and then merge into one in the median-ventral part of the body, thus appearing in a “T” shape. The merged vas deferens extends ventrally to the common gonopore at the bottom of the copulatory atrium (ca) ( Figs. 1 View FIGURE 1 G, 3B–C). The male copulatory organ is in tubular shape.
A pair of elliptical-shaped ovaries (o) (84 µm×33 µm) is situated ventrally at both sides of the copulatory atrium (ca), forming a “V” shape ( Figs. 1 View FIGURE 1 F–G, 2F, 3B). Two oviducts (ov), 56–75 µm in length, originate from both sides of the ovaries, extend to the common oviduct (cov) that is 27–40 µm in length, and finally enter the copulatory atrium ( Figs. 2 View FIGURE 2 F–G, 3B–C). Two white vitellaria are situated at both sides of the intestine (i). They are strip-like with the length identical to that of the intestine ( Figs. 1 View FIGURE 1 A, 3A). Vitelline ducts from both sides merge at the trailing edge of the common oviduct and enter the copulatory atrium via the common oviduct.
Habits and reproduction. The flatworms were fed daily with Daphnia sp. In mature individual, there is usually a red color cocoon behind the pharynx. During reproduction period, the mature individual lays one oval cocoon per day. The cocoon is in brown-red color, 434–503 µm in length and 325–387 µm in width ( Figs. 1 View FIGURE 1 B–C). The incubation period was about 14 days, and each cocoon could hatch one larva. In total, 7 cocoons have been observed in a 12-well plate, with each well contains a single cocoon. The larvae could be maintained for up to 12 days post-hatching and developed into juvenile stage, but were unable to reach sexual maturity.
Habitat. Aquatic plants were introduced into the artificial lake from the suburb of Foshan, Guangdong in 2006. In 2011, a small artificial wetland was constructed on the islet in the center of the lake in order to purify the polluted water. Although no flatworm was found in the lake, the B. sinensis n. sp. was found in a cement filtering basin of the wetland. During collection, the ambient water was limpid, with a small quantity of spirogyra on the basin wall. There were abundant invertebrates, including several kinds of Rhabdocoela and Tricladida in the basin ( Lu et al. 2014). In 2013, this species disappeared, mainly due to water pipe blockage and the subsequent water quality deterioration. However, in early 2016, the lake water was treated and lotus was planted. Seven more individuals were collected on July 16, 2016 at the same sampling site.
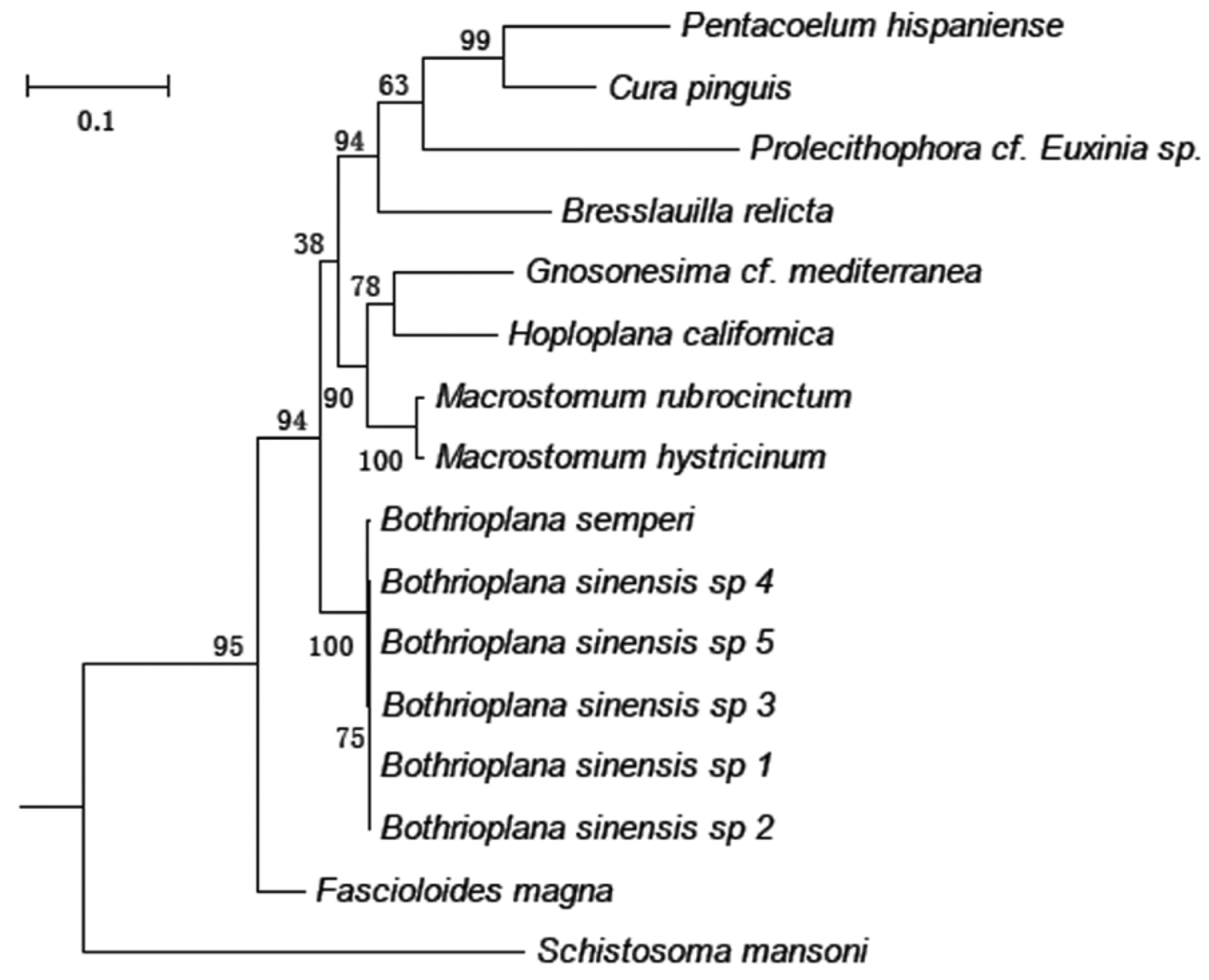
Phylogenetic analysis. The 18S rDNA phylogenetic analysis using Maximum-Likelihood (ML) and Neighbor-Joining (NJ) methods showed that Bothrioplana sinesis n. sp. specimens 1–5 clustered together and formed a well-supported clade with another species within the genus, namely B. semperi . This new species is related more closely to species from the family Macrostomidae and Fasciolidae , than to those of Gnosonesimidae Letoplanidae, Graffillidae , Bdellouridae , Dugesiidae , or Schistosomatidae .
Discussion. To date, 7 orders of turbellarian have been reported in China, including Rhabdocoela , Tricladida , Polycladida, Temnocephaloda , Macrostomida , Lecithoepitheliata and Prolecithophora . The Bothrioplana sinensis n. sp. characterized in this paper represents a newly recorded order in China and the second identified species in the genus Bothrioplana .
Only two species in the genus Bothrioplana , namely B. semperi ( Braun 1881) and Bothrioplana . sp. ( Kawakatsu & Mack-Fira 1975), have been recorded so far. Due to the incomplete description of Bothrioplana . sp., comparison between the new species and Bothrioplana . sp. was impracticable. As for B. semperi , the specimen was first discovered in Tartu, Estonia ( Braun 1881), and then found in Karkonosze, Poland ( Zacharias 1886), Lake Geneva, Switzerland ( Rixen 1961), Loch Lomond, Scotland, United Kingdom ( Young 1976) and Northern Australia ( Sluys & Ball 1985), respectively. The head of B. semperi is in a square shape with 2 pairs of sensory pits. A pair of round testes is lacated at both sides of the center of pharynx ( Young 2001). According to Sluys and Ball (1985), the male copulatory system is composed of vas deferens, seminal vesicle and granular vesicle. The granular vesicle enters the copulatory atrium from dorsal side. The female copulatory system has genitor-intestinal duct. In most cases, B. semperi does not produce sperm. Even though the sperm are produced, they are abnormal and sterile ( Dahm 1951).
In this study, 32 Bothrioplana sinensis n. sp. specimens were examined, including 16 mounted specimens and 4 serial section specimens. The head of the new species is arc-shaped. A pair of spherical-shaped testes is located in both sides posterior to the pharynx. The male copulatory system has no granular vesicle. The mature individuals have testes with a large number of mature sperm. The female copularoty system has no genitor-intestinal duct. The paired oviducts and the paired vitelline ducts fuse into a common oviduct ventral-posteriorly and enters the copulatory atrium. The immature individuals have ovaries but no testis, suggesting that the female copulatory system develops earlier than the male’s. Its morphological characteristics are distinct from those of the B. semperi . In addition, the molecular phylogenic evidence also supports the classification of this species. Therefore, it is evident that B. sinensis n. sp. described in this study is a new species within the genus Bothrioplana .
The discovery of well-developed testes and fully functioning sperm in the specimens of Bothrioplana sinensis n. sp. is a highly interesting finding from a biological standpoint, as B. semperi has been described as an obligate parthenogen, employing a distinct and only mode of reproduction termed “dioogamy” (see Martin-Duran and Egger 2012). The B. semperi were occasionally found to demonstrate testes and a copulatory organ, however, even the mature individuals containing a rudimentary male system are incapable of normal spermatogenesis or producing fully functioning sperm ( Laumer et al. 2015). Another interesting finding is B. sinensis n. sp. and B. semperi form a clade that is closely related to Fascioloides magna , which is a parasitism species belongs to the subphylum Neodermata. This result is in good agreement with some of the previous studies ( Baguñà & Riutort 2004, Laumer et al. 2014; Laumer et al. 2015).
Since the aquatic plants of the sampling site were introduced from Foshan, Guangdong, it is possible that Bothrioplana sinensis n. sp. was originated from wetlands in Foshan. In addition, B. semperi is a cosmopolitan species ( Tyler et al. 2012) and is suggested to be capable of long distance dispersal. Therefore, the distribution of this new species within the region warrants further study.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |