Coeliades forestan forestan (Stoll)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6788694 |
|
persistent identifier |
https://treatment.plazi.org/id/1476B03C-FFF5-1B1E-FF13-F8BBB98CFEE8 |
|
treatment provided by |
Felipe |
|
scientific name |
Coeliades forestan forestan (Stoll) |
| status |
|
Coeliades forestan forestan (Stoll) View in CoL ( Figures 18–30 View FIGURE 18 View FIGURE 19 View FIGURE 20 View FIGURE 21 View FIGURE 22 View FIGURE 23 View FIGURE 24 View FIGURE 25 View FIGURE 26 View FIGURE 27 View FIGURE 28 View FIGURE 29 View FIGURE 30 )
The nominate subspecies occurs throughout tropical Africa, except Madagascar where it is replaced by subspecies arbogastes Guenée. This is the most common and widespread of the genus in Kenya, its range extending into some of the driest country in the North. The adult is most conspicuous, at least in the rainy season, in the drylands between Nairobi and the Coast. For example, van Someren (1939) reports it very numerous at low altitudes in the Chyulu Hills at c. 900m (3,000 ft.), and extending up to c. 1800m (6,000 ft.), and Sevastopulo (1974) found it common in and around Makadara Forest in the Shimba Hills throughout the year. However, caterpillars can be found all over the country on various food plants, for example I have found caterpillars several times in Kakamega Forest on Combretum sp. , a sprawling vine, but over many visits have never seen adults in the forest. This is also true of several other localities which I have only visited once or twice.
Adult behaviour
This species will fly and feed throughout the day—from 07.00h (Bahari Beach, north of Dar es Salaam) until dusk (Mazoe Dam, north of Harare)—a habit also noted by Trimen (1889) and Dickson & Kroon (1978). Migdoll (1988) states that it is more frequently seen in the early morning and late afternoon. Flight is rapid, and C. forestan is not readily separable from Pyrrhiades a. anchises and C. pisistratus in flight, except sometimes when close up or when at rest ( Figure 18 View FIGURE 18 ). I have observed oviposition at 15.30h at Diani Beach and in the morning at Kibwezi Forest. On the latter occasion (23 Dec 1990, 90/127), one shoot of Combretum sp. (093) had three ova: two unhatched and one hatched, two on the abaxial surface of young leaves, and one against a bud on the shoot. Oviposition at this sort of density will lead to caterpillar density and damage such as that shown in Figure 22 View FIGURE 22 (left) of the same food plant at the same location.
Coeliades f. forestan View in CoL is an avid visitor to flowers, and I have seen it at Lantana camara View in CoL , wild sunflower, Vernonia sp. and several other species; of these L. camara View in CoL seems particularly preferred. Dickson & Kroon (1978) associate this species with hill tops, but that has not been my experience in Kenya. I have seen C. f. forestan View in CoL at hill tops occasionally, but invariably it has been just passing through. Henning et al. (1997) state that they hilltop, but not for long. Coeliades forestan View in CoL is noteworthy as the first species of Lepidoptera View in CoL reported to use scent-marking. Williams & Woodhall (2006) describe males patrolling a linear territory, stopping briefly on leaves 1–2m above the ground and marking leaves by means of scale covered papilla-like structure extruded from the dorsal side of the terminal abdominal segment. The function of this behaviour has not been established, but is likely to involve repelling other males, or attracting females or both. It is unlikely that C. forestan View in CoL is the only skipper showing this behaviour, but additional observations on the behaviour or the organ used have not been forthcoming as yet.
Food plants
This species is particularly striking for the number of different food plants it uses from different families ( Table 2 View TABLE 2 ), although some of these seem unlikely and would benefit from confirmation, e.g. Peacock’s (1913) record from Brassicaceae . In Kenya, I have recorded it from the species listed in Table 3 View TABLE 3 ( Figures 19–20 View FIGURE 19 View FIGURE 20 ). In addition, on a visit to the Ukiriguru Agricultural Research Institute, near Mwanza, Tanzania, I noted a specimen of C. f. forestan in the insect collection reared at Ukiriguru, 1.vi.1958 on “ C. procera ” by I.A.D. Robertson (ref. 3933). The food plant is only likely to be Calotropis procera (Apocynaceae) .
1 Also found on this food plant near Mangochi, Lake Malawi, Malawi, Jul 2001 .
Caterpillars will not necessarily transfer easily from one plant species to another. For example caterpillars collected on Indigofera tinctoria ( Figure 19 View FIGURE 19 , right) at Diani Beach initially refused to feed on I. arrecta ( Figure 19 View FIGURE 19 , left) from Nairobi (on which I subsequently found a caterpillar in my Nairobi garden), or on another Indigofera sp. (060) from Ngong Forest. Conversely however, caterpillars collected on I. arrecta (Magadi Road, 89/79) and Combretum sp. A (Kibwezi Forest, 90/10) readily accepted I. tinctoria grown at Nairobi.
The life history has been described by Aurivillius (1925), described and figured by Trimen (1889), Murray (1932) and Henning et al. (1997), and illustrated in detail by G.C. Clark (in Dickson & Kroon 1978) from South Africa.
Ovum
Ova are laid singly on the underside of leaves (sometimes upper side in the case of Indigofera spp. ) or the young stems of the food plants. Normally oviposition on the leaf lamina under surface is near the margin, but on a large leaf of Terminalia catappa , an ovum was once found on the midrib. The ovum ( Figure 21 View FIGURE 21 ) is creamy white, 0.9 x 0.6mm wide x high, with 15–16 ribs; microsculpture similar to P. a anchises . It takes about 9 days to hatch according to G.C. Clark (in Dickson & Kroon 1978, Plate 1) in South Africa.
Leaf shelters
There is some variation in the detail of the leaf shelters, particularly the third and fourth shelters, linked to the texture and dimensions of the leaves of the varied host plants.
On the large, thin, soft leaves of Quisqualis indica the first shelter is made by cutting two ¾ oval flaps from each side of a major vein, and folding them upwards to make a rounded shelter on top of the leaf 4–5mm long x 2mm high; in the example examined, one flap cut from the edge of the lamina and the other from within the lamina. Where the shelter is made well within the lamina, just one three-quarter oval major cut would be needed for each flap making this a two–cut shelter, whereas if the shelter were made on the mid-rib near the leaf tip (as for P. a. anchises above) it would probably be a four–cut shelter. The example described above would be a three–cut shelter in the terminology of Greeney & Jones 2003). These are all functionally the same shelter, but formed in different ways depending upon where on the leaf the shelter is made, and how big the leaf is. The shelter is subsequently skeletonised by the feeding of the young caterpillar, perhaps because the caterpillar can thereby avoid leaving the shelter and being exposed to predators and other risks. The stage 2 leaf shelter is similar but larger, 11mm long, 5 mm high and 5 mm wide; again the shelter is skeletonised by the feeding caterpillar. The stage 3 shelter is made using the mid-rib adjacent to the bridge, either folding one flap onto the top of leaf (a type 4 one–cut shelter or a type 5 two–cut shelter), or folding two flaps together, similarly to the stage 1 and 2 shelters. Similar stage 1 shelters are made on the thicker, tougher leaves of Combretum sp. A ( Figure 22 View FIGURE 22 ) and probably other Combretum spp.
On the large thick leaves of Terminalia catappa the first three shelters are similar ( Figure 23 View FIGURE 23 , left), but stage 4 leaf shelters are also made by folding a broad leaf margin over or under in a type 1 no–cut shelter ( Figure 23 View FIGURE 23 , right), e.g. Diani Beach (91/49), Lake Malawi, Malawi (01/102).
On the tiny leaflets of Indigofera spp. ( Figure 24 View FIGURE 24 ) the first shelter is a type 6 four–cut shelter similar to those on thicker leaves, because the leaflets are so small. For the stage 2 shelter, two or three leaflets of one leaf are spun together, and for the stage 3 shelter, leaflets of two or more leaves are spun together—both type 2 multi–leaf shelters.
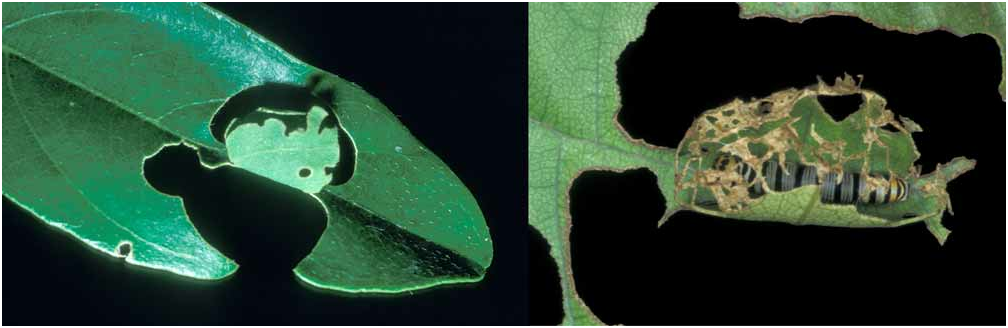
The first occasion on which I found caterpillars of C. forestan was on Lonchocarpus bussei in Arabuko Sokoke Forest on 23 Sep 1989 (89/85). A 9mm caterpillar was found in stage 1 leaf shelter made by cutting two ¾ ovals from each side of the mid-rib, and folding them upwards; one oval reached the leaf lamina, but the other didn’t, thus making this effectively a three–cut shelter; the edge of the flaps had been eaten so as to make them irregular, and at least one small perforation was eaten in one flap ( Figure 25 View FIGURE 25 left). A final instar caterpillar had made a simple shelter between two leaves, the lower slightly convex to make a gap between them—a type 2 multi–leaf shelter. A similar stage 3 shelter containing a fifth instar caterpillar was observed on Combretum sp. B at Kakamega Forest (91/34), except that one of the leaves was of an adjacent Rubus sp. (Rosaceae) .
Caterpillar
There are five caterpillar instars in this species, unlike some other Coeliadinae treated here which may have six or seven instars. Caterpillars are variable, particularly with regard to the size of the black spots on the head. Caterpillars on Combretum sp. A in Kibwezi Forest, always had the head marked heavily with black spots often conjoined ( Figure 28 View FIGURE 28 , lower row), especially in the first four instars ( Figure 26 View FIGURE 26 ), whereas those on Indigofera tinctoria at Diani Beach were lightly marked ( Figure 28 View FIGURE 28 , upper row); samples from other localities were too small to generalise in this way, but tended to be intermediate in markings. Henning et al. (1997) illustrate a final instar caterpillar similar to those treated here, with a lightly marked head comparable to Figure 28 View FIGURE 28 (upper middle).
Instar 1. The newly hatched caterpillar (28/3) has the head light brown, very diffuse rows of conjoined spots; darker laterally and on posterior margin; 0.8 x 0.7mm wide x high (n=7); T1 green with dark transverse dorsal plate; body green.
Subsequent instars ( Figure 26 View FIGURE 26 ) increasingly resemble instar 5 (below). The head capsules measure: instar 2, 1.3 x 1.3mm (n=6); instar 3, 1.6 x 1.6mm (n=11); instar 4, 2.5 x 2.5 mm (n=22) wide x high; most caterpillars in the early instars have the dorsal row of spots overlapping ( Figure 28 View FIGURE 28 ), but in the latter and final instars these become increasingly separated in some individuals, but not in others ( Figures 27–28 View FIGURE 27 View FIGURE 28 ).
Instar 5 ( Figures 27–28 View FIGURE 27 View FIGURE 28 ). Individual 88/85A was collected on Lonchocarpus bussei in Arabuko Sokoke Forest on 23 Sep 1989, and went on to pupate on 3 Oct. The following description was prepared on the day of collection, when the caterpillar measured 31mm. Head 4.1 x 4.8mm wide x high (compared to an average of 4.2 x 4.2mm, n=17), light tan brown; two rows of black spots across face, the upper row of six with the four central spots oval, extended dorsally, the outer spots smaller, round and slightly lower; the lower row of five with three intermediate spots in centre, and smaller spots laterally and slightly ventrally; posterior margin narrowly black. T1 anterior 2/3 black, posterior 1/3 white. T2–T3 anterior ½ black, posterior ½ white; T3 with a narrow black transverse line in white band. A1–A7 anterior 1/3 black, posterior 2/3 white, with 3 or more transverse narrow black lines; an elongate transverse white streak dorsolaterally in black section, just posterior to middle of black area; sublaterally a white longitudinal line through spiracles; below this line greyish white with obscure darker markings. A8 anterior ½ black, posterior half white, orange-tan dorsolaterally; a single narrow black line in white band. A9 anterior margin black, wider dorsally; posterior to this a white band, angled towards anterior margin laterally, where it becomes orange-tan; posterior to this a black band to posterior margin, except at dorsum which is white. Shortly before pupation the white areas acquired a green tint and the ventrolateral areas a yellow tint.
Pupa
The final shelter may be formed from the last leaf shelter, or by folding under (or sometimes over) the margin of a large leaf such as those of Terminalia catappa ( Figure 23 View FIGURE 23 , right), or between two leaves, one above the other as I have seen on Combretum sp. A .
The pupa of individual 89/8A was formed 3 Oct 1989 and a male emerged 17 Oct; it was described on 12 Oct ( Figure 29 View FIGURE 29 , above). 24mm; dull green covered with white waxy bloom; short slightly upturned frontal spike, 1mm; spiracle T1 an erect blunt spike, black anteriorly and posteriorly; other spiracles black, but small and not conspicuous. Between the eyes a row of four black spots, with the widest gap in the middle. Wings pale under wax bloom; spot at end cell F. Underside of cremaster and the outline of a heart shape anterior to it, black.
There is minor variation in the black markings of the pupa. Thus, the pupa of individual 90/10F collected on Combretum sp. A at Kibwezi Forest had additional black spots: at the base of space 4 F, at base of proboscis, middle of leg of T1; as well as the abdomen having small patches bare of wax covering. Similarly, individual 90/10B collected on the same occasion had a black tip to the frontal spike, and black streaks on F costa near base, and vein 4 near base ( Figure 29 View FIGURE 29 , below). Henning et al. (1997) illustrate a pupa, but in the dorsolateral view the degree of spotting cannot be seen.
I would expect pupation to be in these final shelters, although I have only found empty pupal cases in the field on T. catappa (twice). In captivity, the pupa is not normally formed in a leaf shelter, but often on the lid or between a leaf and the side of the container. This coupled with the fact that I have seldom found pupae in leaf shelters in the field leads me to suspect that pupae may also be formed elsewhere, probably amongst leaves or debris on the ground.
From material reared through at Nairobi, I have recorded 13 pupal durations which averaged 13.5 days with a range of 9–18 days.
Natural enemies
One of four ova collected 9 Jul 1991 at Diani Beach on Indigofera tinctoria was parasitized by a solitary black egg parasitoid (91/53).
The caterpillars are attacked by a braconid parasitoid which paralyses third and fourth instar caterpillars about to moult. The parasitoid larvae feed externally and then spin cocoons as a group ( Figure 30 View FIGURE 30 ). I have reared this parasitoid from C. f. forestan on Indigofera arrecta in Nairobi (90/4), and Combretum sp. A at Kibwezi Forest (90/10A). The former produced nine males and one female, and the latter six males and nine females. What appears to be the same species was reared from P. a. anchises and Coeliades pisistratus . The biology and form of the cocoons appear to match very closely to those of Bracon spp. (e.g. Bennett 1960).
I also reared a solitary ichneumonid larval–pupal parasitoid from this host once. A 13mm caterpillar collected on Combretum sp. A at Kibwezi Forest (90/10D), 21 Jan 1990, pupated 11 Feb, and an ichneumonid wasp emerged 16 Mar.
Discussion
There seems no reason not to interpret these observations as representing a single highly polyphagous species, with a range of variation in caterpillar head markings from almost none, to very heavy spots running into each other. If the extremes, i.e. very light spots on Indigofera tinctoria on the coast and very heavy spotting on Combretum paniculatum in the South Nyambani Hills, had been examined in isolation, it would have been tempting to see them as separate species, but the range of continuous variation found on a variety of food plants shows otherwise. Clearly, interpretation of small numbers of isolated specimens needs to be done with caution.
TABLE 2. Recorded food plants of Coeliades forestan forestan. Localities preceded by a question mark reflect a food plant record from a national or regional work, which may or may not be based on observations in that country or region. What appear to be repeat records from other regions may also be listed against an earlier record.
| Family Food plant | Locality | Reference |
|---|---|---|
| Apocynaceae | ||
| Marsdenia ?‘ senegalensis ’1 | East Africa | van Someren 1974 |
| Marsdenia schimperi (= Dregea schimperi ) | East Africa | van Someren 1974 |
| Marsdenia or Dregea sp. | ?Kenya | Sevastopulo 1975; Henning et al. 1997 |
| Brassicaceae | ||
| Brassica oleracea (Gongylodes group) kohlrabi or German turnip | Nigeria | Peacock 1913; Smith 1965 |
| Chrysobalanaceae | ||
| Parinari excelsa (= curatellifolia) | Southern Africa | Pringle et al. 1994; Henning et al. 1997 |
| Combretaceae | ||
| Combretum sp. | Malawi | Gifford 1965 |
| ?Southern Africa | Pinhey 1965 | |
| ?East Africa | Sevastopulo 1975 | |
| Combretum apiculatum | South Africa | Murray 1932, 1959; Dickson & Kroon 1978; Migdoll 1988; Pringle et al. 1994; Henning et al. 1997 |
| Combretum bracteosum | South Africa | Murray 1932, 1959; Dickson & Kroon 1978; Migdoll 1988; Pringle et al. 1994; Henning et al. 1997 |
| Combretum paniculatum | East Africa | van Someren 1974 |
| Combretum racemosum | Côte d’Ivoire | Vuattoux 1999 |
| Quisqualis indica | Uganda (Kampala) | Sevastopulo unpublished2 |
| Quisqualis sp. | ?Southern Africa | Henning et al. 1997 |
| Terminalia catappa | Mauritius | Williams 1989; Davis & Barnes 1993 |
| Terminalia | ?East Africa | Sevastopulo 1975; Henning et al. 1997 |
| Triaspis odorata | Côte d’Ivoire | Vuattoux 1999 |
| Fabaceae | ||
| Canavalia ensiformis (= gladiata) | Malawi | Smee 1944 |
| Kenya, Uganda | Le Pelley 1959 | |
| Mauritius | Williams 1989; Davis & Barnes 1993 | |
...... continued on the next page
TABLE 3. Food plants of Coeliades forestan forestan recorded by the author in Kenya.
| Family Food plant | Locality | Month(s) |
|---|---|---|
| Combretaceae | ||
| Quisqualis indica | Diani Beach | Dec |
| Terminalia catappa | Diani Beach & Mtwapa1 | |
| Combretum paniculatum | South Nyambani Hills | Apr |
| Combretum sp. A (081) | Kibwezi Forest | Jan, Mar, Jun, Dec |
| Combretum sp. B (093) | Kakamega Forest | Jun, Jul |
| Fabaceae | ||
| Indigofera tinctoria | Diani Beach | Mar, Jul |
| Indigofera arrecta | Magadi Road | Oct |
| Nr Kisii (Kadera God Forest) | Aug | |
| Nairobi | Jan | |
| Indigofera . sp. | Kakamega | May, Nov |
| Lonchocarpus bussei | Arabuko Sokoke Forest | Sep |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Coeliades forestan forestan (Stoll)
| Cock, Matthew J. W. 2010 |
Coeliades forestan
| Stoll 1782 |
C. forestan
| Stoll 1782 |
Lepidoptera
| Linnaeus 1758 |