Pithecopus gonzagai, Andrade & Haga & Ferreira & Recco-Pimentel & Toledo & Bruschi, 2020
|
publication ID |
https://doi.org/10.5852/ejt.2020.723.1147 |
|
publication LSID |
lsid:zoobank.org:pub:400FEC6E-8E57-4207-A0F9-2AC4F5290FE8 |
|
DOI |
https://doi.org/10.5281/zenodo.4657736 |
|
persistent identifier |
https://treatment.plazi.org/id/573D7AF0-0CB0-4D96-989E-EA3286190CB8 |
|
taxon LSID |
lsid:zoobank.org:act:573D7AF0-0CB0-4D96-989E-EA3286190CB8 |
|
treatment provided by |
Valdenar |
|
scientific name |
Pithecopus gonzagai |
| status |
sp. nov. |
Pithecopus gonzagai sp. nov.
urn:lsid:zoobank.org:act:
Figs 2–3 View Fig View Fig ; Tables 1–2 View Table 1 View Table 2
Phyllomedusa nordestina View in CoL (only specimens from locations north of the SFR in the following studies) – Caramaschi 2006: 176–177 View Cited Treatment , fig. 6. — Faivovich et al. 2010: 261, fig. 4, table 2. — Loebmann & Haddad 2010: 256, fig. 3, table 2. — Silva et al. 2010: 340, fig. 2, tables 1–2. — Toledo & Batista 2012: table S1. — Brand et al. 2013: 7065. — Neiva et al. 2013: 140. — Pinto et al. 2013: 656. — Toledo et al. 2015: 88, fig. 2. — Valencia-Aguilar et al. 2015: table 1. — Röhr et al. 2020: 653, fig. 2.
Pithecopus nordestinus View in CoL – Duellman et al. 2016: 91 (GenBank: GQ366016 View Materials , GQ366091 View Materials , GQ366143 View Materials , GQ366330 View Materials ). — Dubeux et al. 2019: table 1; 2020: 15, figs 2, 10. — Silva et al. 2020: 165–172, fig. 1, tables 1, S1.
Diagnosis
Pithecopus gonzagai sp. nov. is assigned to the genus Pithecopus (former Phyllomedusa hypochondrialis species group; Caramaschi 2006) by the following set of characters: (1) small body size; (2) dorsolateral macroglands ( sensu Antoniazzi et al. 2013) indistinct; (3) smooth skin on back and granulose on belly; (4) fingers and toes long and slender with terminal discs poorly developed; and (5) grasping (opposable to the others) finger I and toe I. Pithecopus gonzagai sp. nov. differs from the highland species of Pithecopus by the (6) lack of the reticulate pattern on flanks, and (7) head width smaller than 11.2 mm.
Etymology
The specific name honours Luiz Gonzaga do Nascimento, better known as Luiz Gonzaga. He was a Brazilian singer, songwriter, musician, poet and one of the most influential figures of Brazilian popular music in the twentieth century. Luiz Gonzaga has been credited for presenting the rich universe of northeastern musical genres to the rest of the country. He was born and raised in the municipality of Exu, state of Pernambuco, Brazil. Pithecopus gonzagai sp. nov. also occurs in the state of Pernambuco, which is equally its type locality.
Type material
Holotype
BRAZIL • ♂ ( Fig. 2 View Fig ); north-eastern Brazil, state of Pernambuco, municipality of Limoeiro ; 7°52′29.0″ S, 35°27′01.1″ W; 150 m a.s.l.; 16 May 2011; D.P. Bruschi, M.A. Passos and Jonatha Lima leg.; ZUEC 19685 View Materials . GoogleMaps
Paratopotypes
BRAZIL • 23 ♂♂; same collection data as for holotype; ZUEC 19664 View Materials to 19668 View Materials , 19670 View Materials to 19684 View Materials , 19686 View Materials to 19688 View Materials GoogleMaps • 4 ♀♀; same collection data as for holotype; ZUEC 19661 View Materials to 19663 View Materials , 19669 View Materials GoogleMaps .
Other material examined
BRAZIL – Pernambuco • Limoeiro ; ZUEC 19661 to 19688 • Bom Conselho ; ZUEC 19617 , 19619 , 19622 to 19625 , 19628 to 19633 • Caruaru; ZUEC 19610 to 19616 • Poçṳo ; ZUEC 19638 , 19640 to 19644 , 19646 , 19649 to 19650 , 19654 • Recife ; ZUEC 19655 to 19660 . – Alagoas • Pilar ; ZUEC 19573 , 19575 , 19581 to 19582 , 19584 , 19587 , 19590 to 19591 , 19593 to 19594 • Rio Largo ; ZUEC 19773 , 19775 , 19777 , 19779 , 19781 to 19783 , 19785 to 19787 • S ṳo Miguel dos Campos ; ZUEC 19565 to 19571 • Sṳo Miguel dos Milagres ; 19595 to 19597 , 19599 to 195604 , 19606 to 19608 • Satuba ; ZUEC 18627 to 18633 , 18636 to 18638 . – Paraíba • Araruna ; ZUEC 19788 , 19791 , 19793 , 19841 to 19842 , 19848 , 19854 • Cabaceiras ; ZUEC 19689 to 19692 , 19697 to 19699 , 19702 , 19704 to 19708 , 19710 to 19711 • Campina Grande ; ZUEC 19713 to 19722 , 19724 , 19729 , 19732 , 19733 , 19734 • Joṳo Pessoa ; 19726 to 19728 , 19735 to 19740 , 19743 • Mamanguape ; ZUEC 19747 , 19748 , 19751 , 19753 , 19754 to 19756 . – Rio Grande do Norte • Macaíba ; ZUEC 19817 , 19818 , 19825 , 19828 , 19829 , 19863 • Sṳo Paulo do Potegi ; ZUEC 19805 , 19811 , 19834 , 19846 , 19851 , 19853 .
Description
Holotype
General aspect slender ( Fig. 2 View Fig A–B); snout truncate in dorsal and lateral views ( Fig. 2 View Fig C–D). Head wider than long; loreal region slightly concave; canthus rostralis rounded, smooth; nostrils small, subcanthal, placed latero-frontally, closer to snout tip than to eyes; internarial distance longer than eye-nostril distance and tympanum diameter, but smaller than eye diameter; eyes latero-frontally positioned; tympanum nearly circular, with annuli undefined at superior border; tympanum diameter less than half of eye diameter; supratympanic dermal fold present, beginning on right side of tympanum and ending near insertion of arm; dorsolateral macrogland indistinct; no external vocal sac; tongue nearly ovoid, free posteriorly, longer than wide, without pigmentation on base; vomerine teeth absent; choanae small, located laterally, slightly rounded. Upper arm thin and forearm robust; no finger webbing; comparative finger length when adpressed I<II <IV<III ( Fig. 2E View Fig ); finger discs poorly developed; finger I enlarged at base; nuptial asperity covering most of dorsal surface of finger I, except tip; palmar tubercles poorly developed, subarticular tubercles developed, barely distinguishable from adjacent supernumerary tubercles ( Fig. 2E View Fig ); inner and outer metacarpal tubercles poorly developed; comparative toe length when adpressed II <III <I <V<IV ( Fig. 2F View Fig ); toe webbing absent; plantar callosities poorly developed, inner and outer metatarsal tubercles poorly developed; subarticular tubercle developed, single and rounded; supernumerary tubercles rounded and poorly developed ( Fig. 2F View Fig ); legs slender; dorsal skin smooth; ventral skin granulated on belly, throat and thigh, smooth on tibia, tarsus and foot. Chest and right thigh slightly damaged ventrally due to tissue sampling. Cloacal region moderately granulated.
Measurements of holotype (mm)
SVL = 32.7, HL = 6.9 (21.1 % of SVL), HW = 9.8 (30.0 % of SVL), AGL = 15.6 (47.7 % of SVL), ED = 3.7 (11.3 % of SVL), TD = 1.2 (3.7 % of SVL), END = 2.2 (6.7 % of SVL), IND = 3.2 (9.8 % of SVL), UAL = 5.6 (17.1 % of SVL), FAL = 7.1 (21.7% of SVL), HAL = 8.1 (24.8 % of SVL), TGL = 12.7 (38.8 % of SVL), TL = 12.8 (39.1 % of SVL), TAL = 8.3 (25.4% of SVL) and FL = 10.9 (33.3 % of SVL).
Colouration in life
Dorsum of head and body green. Vertebral line on back present in some individuals ( Fig. 3C View Fig ), but absent in all individuals of the type series. Loreal region and eyelids green. Eyes outlined with thin white line. Edge of jaw bordered by black line. Green dorsal region delimited by white dorsolateral line extending from mouth end until around middle of axilla-groin length. Same white line also observed in distal parts of forelimbs, extending from elbow to end of finger IV. Black dorsolateral line (below white line) delimits ventral region; same line also found on forearms, tibia and foot portion. Anterior and posterior surfaces of upper arm, thigh and tibia, extending partially onto foot, orange with well-defined vertical black stripes. Forearm anterior surface extending to hand coloured orange with well-defined vertical black stripes. Orange inguinal region with well-defined vertical black stripes. Green dorsal surface extending onto all limbs: arm, forearm, thigh, knee and tibia; green dorsolateral surface of tarsus and foot ( Fig. 3 View Fig A–C).
Variation in the type series
The specimens ZUEC 19661, 19663, 19667, 19669–70 and 19677–82 have slightly darkened blotches on back. The specimens ZUEC 19661–3, 19667 and 19669 have slightly darkened dots on back. The specimens ZUEC 19661–3, 19669–70, 19678–80 and 19688 have a well-defined dark stripe on edge of jaw (angular region). The specimens ZUEC 19664, 19668–9, 19673, 19675, 19680 and 19684 have slightly darkened terminal discs. Females larger than males, with robust body and lacking nuptial pads.
Differential diagnosis
Pithecopus gonzagai sp. nov. is promptly distinguished from P. centralis , P. megacephalus , P. ayeaye , P. oreades and P. rusticus by the absence of the reticulate pattern of colouration on flanks ( Bokermann 1965; Lutz 1966; Brandṳo 2002; Caramaschi 2006; Bruschi et al. 2014). Pithecopus gonzagai sp. nov. is distinguished from P.centralis and P. megacephalus by its smaller head width ( 8.9–11.2 mm in P. gonzagai sp. nov. vs 12.2–14.5 mm, combined values for other species). Also, P. gonzagai sp. nov. differs from P. ayeaye , P. megacephalus , P. oreades and P. rusticus ( 9.9–12.7 mm, combined values) by its smaller head length ( 6.2–8.9 mm). The new species is distinguished from P. centralis and P. megacephalus (3.0– 3.5 mm, combined values) by its smaller eye-nostril distance ( 1.8–2.8 mm) ( Bokermann 1965; Lutz 1966; Brandṳo 2002; Caramaschi 2006; Bruschi et al. 2014). Pithecopus gonzagai sp. nov. is also distinguished from P. centralis (40.0–42.0 mm) by its smaller SVL ( 28.5–37.8 mm) ( Bokermann 1965). The new species is distinguished from P. rusticus by the absence of the slightly reticulated pattern on the palpebral membrane and the throat region (pattern unique to P. rusticus ; Bruschi et al. 2014).
From its closer relatives (the lowland species), Pithecopus gonzagai sp. nov. is distinguished by being smaller than P. nordestinus , P. azureus and P. hypochondrialis in SVL , head width and length of axillagroin, internarial distance, and upper arm, hand, thigh, tibia and foot lengths (Exact Wilcoxon-Mann-Whitney Test: P <0.01; see Table 3 View Table 3 ). In addition, based on P values obtained from the Exact Wilcoxon-Mann-Whitney Test ( Table 3 View Table 3 ), P. gonzagai sp. nov. is smaller than P. nordestinus in eye-nostril distance ( 1.8–2.8 mm in the new species vs 2.0– 3.1 mm in P. nordestinus ), forearm length ( 6.3–8.8 mm in the new species vs 6.9–9.3 mm in P. nordestinus ) and tarsus length ( 7.9–10.5 mm in the new species vs 8.8–11.1 mm in P. nordestinus ). The new species is larger than P. araguaius in SVL , head length, head width, axilla-groin and eye-nostril lengths, and upper arm, hand, thigh and foot lengths (see Table 3 View Table 3 ). The randomForest model on morphometric traits classified 90% of the males of the new species correctly ( Table 4 View Table 4 ). It was the species with the highest percentage of males classified correctly in comparison with the other four taxa. DAPC based on morphological traits yielded no noticeable discrimination among the new species and P. azureus , P. nordestinus and P. araguaius ( Fig. 4A View Fig ). However, it is possible to notice slight discrimination between the new species and P. hypochondrialis ( Fig. 4A View Fig ), with a greater separation along axis 1 (LD1 = 58%; axis x), but the axis 2 (LD2 = 25%; axis y) also contributes to the separation. Tarsus length (17%), head width (12%), tibia (12%) and foot (11%) lengths mainly accounted for species separation along LD1 ( Fig. 4A View Fig ); while tarsus (25%) and hand (16%) lengths, eyenostril distance (11%) and internarial distance (10%) accounted for the separation along LD2 ( Fig. 4A View Fig ). Morphometric measurements of all examined specimens and the eigenvectors of the DAPC based on morphometric traits are in Supplementary files 3 and 4, respectively.
In comparison with the lowland species, the new species differs from P. palliatus by having the advertisement call with a higher dominant frequency (above 1900 Hz in P. gonzagai sp. nov. vs 1580 Hz in P. palliatus ) and with a single note (= core portion) (double notes in P. palliatus ; K̂hler & L̂tters 1999). Based on the Exact Wilcoxon-Mann-Whitney Test results, the new species can be distinguished from P. hypochondrialis and P. araguaius by having a lower number of pulses per advertisement call (new species: 3.3 ± 0.4 [3–4], P. araguaius : 6.0 ± 0.6 [5–8; P = 3.50 × 10 -3], P. hypochondrialis : 4.2 ± 0.4 [3–6; P = 7.42 × 10 -4]; present study; Haga et al. 2017a) and pulses per core (new species: 3.0 ± 0.0 [3–3], P. araguaius : 6.0 ± 0.5 [5–8; P = 3.50 × 10 -3], P. hypochondrialis : 3.9 ± 0.3 [3–5; P = 2.61 × 10 -6]; present study; Haga et al. 2017a), and shorter core duration (new species: 22.0 ± 4.1 [17–35 ms], P. araguaius : 39.3 ± 5.4 [28–48 ms; P = 3.50 × 10 -3], P. hypochondrialis : 32.0 ± 4.0 [19–61 ms; P = 1.77 × 10 -4]; present study; Haga et al. 2017a). In addition, the new species can also be differentiated from P. araguaius by the lower peak of the dominant frequency of its advertisement call (new species: 2118 ± 63.2 [1969–2391 Hz], P. araguaius : 2540 ± 308 [2240–3316 Hz; P = 4.66 × 10 -3]; present study; Haga et al. 2017a), and from P. hypochondrialis by its shorter inter-pulse interval within the core (new species: 1.8 ± 0.8 [0–4 ms], P. hypochondrialis : 2.0 ± 1.0 [0–7 ms; P = 1.06 × 10 -2]; present study; Haga et al. 2017a).
We were unable to find qualitative or quantitative diagnostic acoustic characters (absence of overlaps) between the new species, P. nordestinus and P. azureus . The randomForest model on acoustic traits was unable to distinguish one male of the new species from those of P. hypochondrialis ( Table 4 View Table 4 ); the other five males were correctly classified. All six males of P. nordestinus were correctly classified. The univariate analyses did not recover differences between calls of the new species, P. azureus and P. nordestinus . In addition, there are overlaps in the acoustic traits of these three species. Among the lowland species, P. araguaius is the more acoustically distinct species ( Fig. 4B View Fig ). The greater separation among lowland species was along axis 1 (LD1 = 91%), while axis 2 contributed much less to the separation (LD2 = 7%). Pulses per core (30%), core duration (22%), isolated pulse (21%) and number of pulses per call (18%) mainly accounted for species separation along axis 1 ( Fig. 4B View Fig ). Eigenvectors of the DAPC based on acoustic traits are in Supplementary file 5.
Phylogenetic inferences and species delimitation test
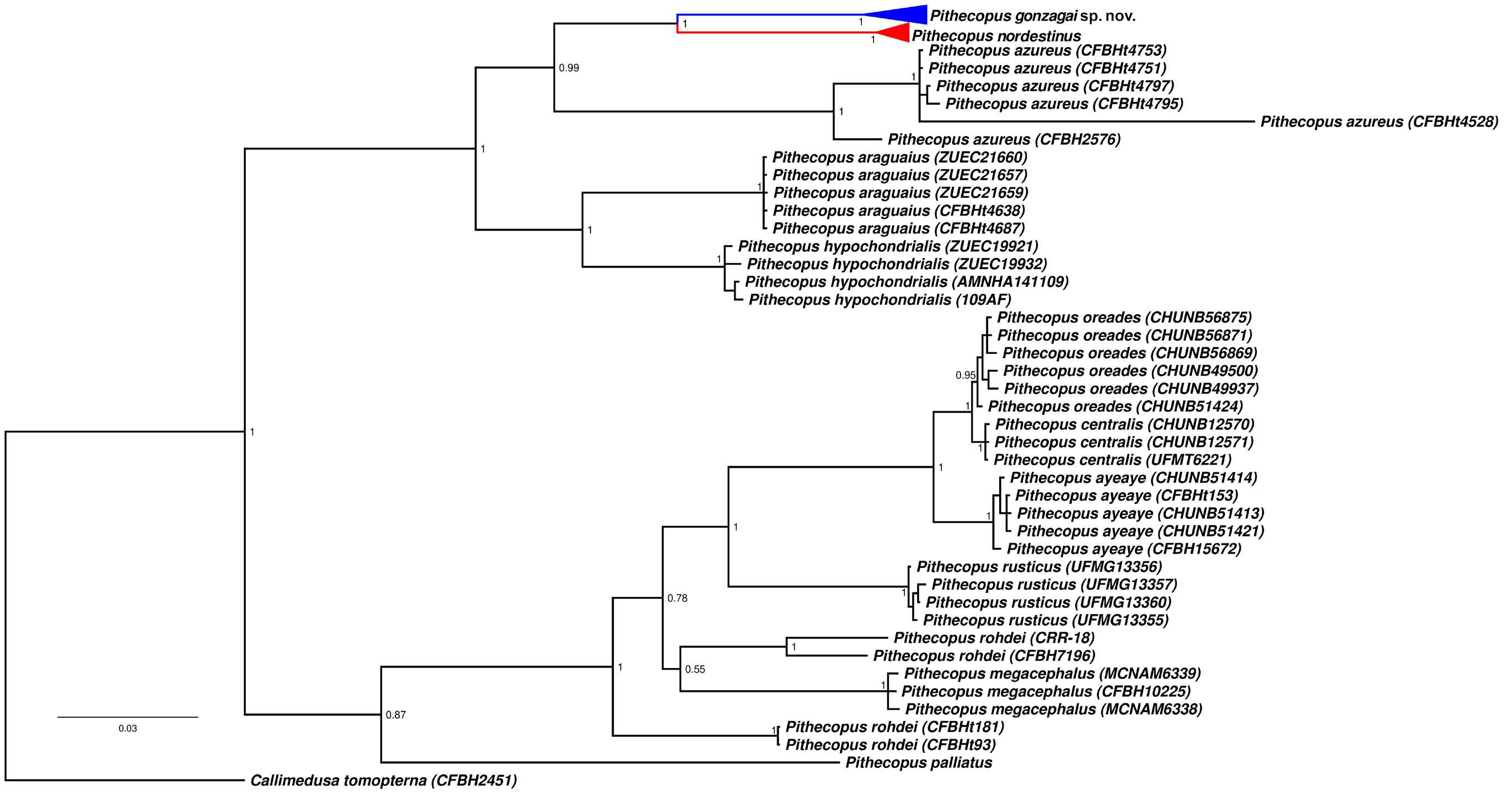
Bayesian inference was congruent with the phylogenetic intrageneric relations recovered by Faivovich et al. (2010), Duellman et al. (2016) and Haga et al. (2017a). In our topologies, we recovered two wellsupported divergent clades (North and South) traditionally assigned to P. nordestinus , coincident with the phylogeographic breaks reported by Bruschi et al. (2019). The paratopotypes of P. gonzagai sp. nov. were recovered nested all with populations from north of SFR while the specimens of P. nordestinus were assembled with populations from south of SFR ( Fig. 5 View Fig , Supplementary file 6). The P. gonzagai sp. nov. + P. nordestinus are sister-group of the P. azureus ( Fig. 5 View Fig , Supplementary file 6).
The single-locus species discovery strategies by GMYC and bPTP approaches were performed in a dataset composed of 148 sequences consisting of 1049 nucleotides from mitochondrial DNA 16S gene, 134 sequences consisting of 892 nucleotides from ND2 and 73 sequences consisting of 422 nucleotides from Siah. Both the GMYC and bPTP identified within the stringent threshold two taxonomic entities, congruent with two main lineages recovered by Bayesian inference (see Fig. 5 View Fig , Supplementary files 6, 7, 8, 9).
Our results recovered same species clusters as shown by Bruschi et al. (2019) in a multi-locus study. GMYC analysis under an estimated ultrametric tree showed the threshold time (16S: -0.0001845922; ND2: -0.01166751; Siah: -0.0002401328) indicating the time all nodes reflect coalescent events; the likelihood of the null model was 1631.647 (16S), 1289.623 ( ND2) and 644.3782 ( Siah) and the maximum likelihood of the GMYC model was 1635.787 (16S), 1291.181 ( ND2), 651.7409 ( Siah). Because the differentiation among samples of P. nordestinus and P. gonzagai sp. nov. based on mitochondrial markers revealed high genetic distances level, the GMYC model suggested 52 entities composed of 32 distinct clusters for 16S, three entities composed of three clusters for ND2 and 11 entities composed of ten clusters for Siah, however, with the exception of two individuals from Areia Branca, no mixture between samples of P. nordestinus and P. gonzagai sp. nov. was recovered in our inferences.
The tree resulting from the bPTP analysis recovered two species from the best ML search, completely separating individuals of the south ( P. nordestinus ) from individuals of the north ( Pithecopus gonzagai sp. nov.). The highest Bayesian solution was similar to the ML tree; however, a total of 118 (16S), 85 ( ND2) and 54 ( Siah) putative entities were recovered by a simple heuristic search. In contrast, a mass sampling can contain individuals from real isolated clades and individuals in different states of structured populations, which may be the cause of the high number of entities found in the results from GMYC and bPTP analyses.
Advertisement call
Six males and 73 advertisement calls of the new species were recorded and analysed. Quantitative call traits are summarized in Table 2 View Table 2 . The advertisement call of P. gonzagai sp. nov. consists of a single pulsed note emitted sporadically ( Fig. 6 View Fig A–B). The calls are generally composed of a main group of pulses (= core portion) ( Fig. 6A View Fig ), which may be followed by one low-amplitude final pulse ( Fig. 6B View Fig ). When present (37 % of analysed calls), the isolated pulses were limited to one and lasted 4–12 ms, separated from the core portion by a long interval of 8–22 ms. The pattern of three-pulsed core without the isolated pulse was more common (63% of analysed calls; Fig. 6A View Fig ), and lasted 17–35 ms. Taking into account the isolated pulses, the call lasted 17–49 ms and has 3–4 pulses. The pulse duration varied from 5–12 ms, emitted at rates of 86–177 pulses / second. The inter-pulse interval (or no interval) within the core lasted 0–4 ms. The peak of dominant frequency varied from 1969 to 2391 Hz ( Fig. 6 View Fig A–B).
Six males and 105 advertisement calls of P. nordestinus (sister species) were recorded and analysed. The advertisement call of the sister species of the new taxon also contains a single pulsed note, with sporadic emission (quantitative call traits are summarized in Table 2 View Table 2 ; Fig. 6 View Fig C–D). Core duration varied from 17 to 33 ms and the intervals between the core and the isolated pulses varied from 6 to 48 ms. The number of pulses per core varied from 3 to 4 pulses, with pulse intervals (or no interval) within the core from 0 to 6 ms. Pulses were arranged in: (1) a three-pulsed core with one isolated pulse (62.9%); (2) a threepulsed core with no isolated pulse (16.2%); (3) a three-pulsed core followed by two isolated pulses, with long inter-pulse interval between them (17.1%; Fig. 6D View Fig ); (4) a four-pulsed core followed by an isolated pulse (2.9%); and (5) a four-pulsed core followed by two isolated pulses, with long inter-pulse interval between them (1.0%). The entire call duration lasted 19–85 ms and has 3–6 pulses, the duration of which varied from 3 to 16 ms, emitted at rates of 91–177 pulses / second. The peak of dominant frequency varied from 1969–2250 Hz ( Fig. 6D View Fig ).
Distribution
Pithecopus gonzagai sp. nov. is known from the type locality and from the 16 municipalities in four north-eastern Brazilian states ( Rio Grande do Norte, Paraíba, Pernambuco and Alagoas), based on molecular, acoustic and morphological evidence. It is possible that all populations occurring north of the SFR can be attributed to this new species, such as the populations from the states of Ceará and Piauí ( Haddad et al. 2013; Roberto & Loebmann 2016); whereas all populations from south of the SFR (states of Sergipe and Bahia) are attributed to P. nordestinus ( Fig. 1 View Fig ).
Natural history
Adult males of P. gonzagai sp. nov. call in the open areas, by the margins of lentic environments (mostly ponds) during the rainy season of the year (which could vary across its distribution). Females lay eggs on leaves over the water bodies ( Fig. 3D View Fig ), from where exotrophic tadpoles hatch and drop into the water. Therefore, the reproductive mode is the number 24 ( sensu Haddad & Prado 2005). Different call types (advertisement, distress, warning and fighting calls) were described and previously attributed to P. nordestinus (see Toledo et al. 2015). However, part of those calls must now be attributed to P. gonzagai sp. nov.: the distress and warning calls described by Toledo et al. (2015) were recorded from individuals sampled in the state of Rio Grande do Norte. Therefore, this population falls within the distribution of P. gonzagai sp. nov., not P. nordestinus . Thus, it is clear that both species ( P. nordestinus and P. gonzagai sp. nov.) have a complex vocal repertoire that should be further explored.
Conservation remarks
Besides corroborating previous observations, we draw attention to a recent governmental act to solve the water supply in semi-arid regions of north-eastern Brazil, the so-called Sṳo Francisco River Transposition Project. Already in progress, the transposition project is diverting water from SFR to temporary rivers and reservoirs of the polygon of droughts (called in Portuguese ʻpolígono das secasʼ) ( Lee 2009). This region is north of the SFR and suffers historically with the effects of prolonged droughts. In this project, there are two canals called the North and the East Axes ( Fig. 1 View Fig ), which pump water from SFR to the driest regions when necessary ( RIMA 2004). We may predict two possible impacts to the regional native fauna, including the present pair of species, P. nordestinus and P. gonzagai sp. nov.: a reduction of the geographic barrier effectiveness and/ or even creation of new artificial barriers elsewhere. Therefore, the whole historical evolutionary dynamics of the species that inhabit this region would be modified. In the case of P. gonzagai sp. nov., its type locality and some others may be affected because they are between the new permanent and artificial rivers and the reduced SFR ( Fig. 1 View Fig ). Therefore, we suggest a long-term genetic survey in this area to monitor the possible effects of the SFR Transposition Project.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pithecopus gonzagai
| Andrade, Felipe Silva de, Haga, Isabelle Aquemi, Ferreira, Johnny Sousa, Recco-Pimentel, Shirlei Maria, Toledo, Luís Felipe & Bruschi, Daniel Pacheco 2020 |
Phyllomedusa nordestina
| Toledo L. F. & Martins I. A. & Bruschi D. P. & Passos M. A. & Alexandre C. & Haddad C. F. B. 2015: 88 |
| Brand G. D. & Santos R. C. & Arake L. M. & Silva V. G. & Veras L. M. C. & Costa V. & Costa C. H. N. & Kuckelhaus S. S. & Alexandre J. G. & Feio M. J. & Leite J. R. S. A. 2013: 7065 |
| Neiva M. & Vargas D. C. & Conceic uo K. & Radis-Baptita G. & Assakura M. T. & Jared C. & Hayashi M. A. F. 2013: 140 |
| Pinto E. G. & Pimenta D. C. & Antoniazzi M. M. & Jared C. & Tempone A. G. 2013: 656 |
| Faivovich J. & Haddad C. F. B. & Baeta D. & Jungferd K. H. & Alvares G. F. R. & Brand uo R. A. & Sheil C. & Barrientos L. S. & Barrio-Amoros C. L. & Cruz C. A. G. & Wheeler W. C. 2010: 261 |
| Loebmann D. & Haddad C. F. B. 2010: 256 |
| Silva G. R. & dos Santos C. L. & Alves M. R. & de Sousa S. D. V. & Annunziata B. B. 2010: 340 |
| Caramaschi U. 2006: 176 |
Pithecopus nordestinus
| Duellman et al. 2016: 91 |
| Dubeux et al. 2019 : table 1; 2020 |
| Silva et al. 2020: 165–172 , fig. 1, tables 1, S1. |