Ornatodorcadion, de Breuning, 1947
|
publication ID |
https://doi.org/ 10.11865/zs.201909 |
|
publication LSID |
lsid:zoobank.org:pub:76D9387A-091B-4D52-840E-8B9C73B56CCC |
|
DOI |
https://doi.org/10.5281/zenodo.5456910 |
|
persistent identifier |
https://treatment.plazi.org/id/127CBD12-FFE4-9A62-FF6B-FE95FD22CDEC |
|
treatment provided by |
Diego |
|
scientific name |
Ornatodorcadion |
| status |
|
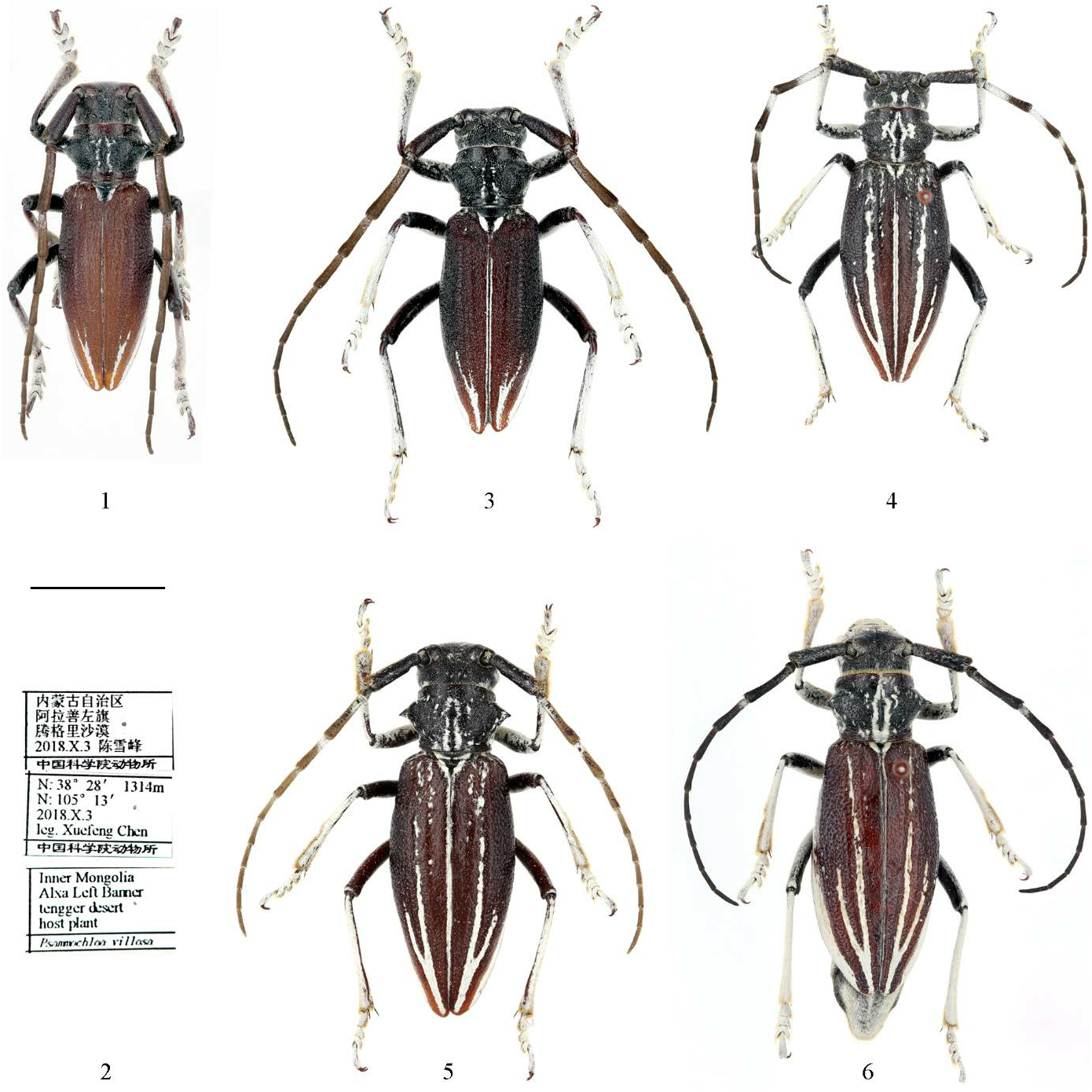
Eodorcadion ( Ornatodorcadion ) zhaoi sp. nov. ( Figs 1–6 View Figures 1–6 )
Type locality. China, Inner Mongolia, Alxa Left Banner, Tengger Desert , elev. 1314 m, 38°28′N, 105°13′E GoogleMaps .
Diagnosis. The new species is close to E. (O.) potanini (Jakovlev, 1889) described from “Ordos” because of the usually similar (red-brown) body color, comparable pronotal and elytral sculpture, and nearly equal antennal length and proportions. But in E. (O.) zhaoi sp. nov., the body is relatively wider (especially in males), more curved laterally, and more attenuated posteriorly. The head is relatively wider, and the cicatrix is considerably obliterated. The elytral carinae are not distinct, the humeral carinae are never roughly sculptured, the external dorsal and humeral elytral stripes are not complete, the marginal elytral stripes are never regularly wide up to the anterior elytral margin, and in females, small traces of the internal dorsal elytral stripes can be visible.
Description. Body length. Male 18.5–23.5 mm, width 6.2–8.0 mm; female 25.4–27.6 mm, width 8.5–9.5 mm. Body reddish-brown including legs and antennae; prothorax, head and ventral side of body considerably darker; head big, wider than anterior pronotal margin; frons about as long as wide in females, while a little narrower in males; lower eye-lobe about as long as gena; clypeus and gena covered with dense white pubescence; frons with irregular white spots; vertex usually with two white stripes; antennae in males a little longer than elytra, surpassing elytral apex by 2 or 3 apical segments; antennae in females reaching elytral apex by last segment; 1st antennal segment with poorly developed cicatrix; 1st segment in males about as long as 3rd, while in females a little longer; other segments much shorter, gradually diminished in length; in females basal halves of 3rd–4th segments ( Fig. 4 View Figures 1–6 ) covered with dense white pubescence (sometimes poorly developed or lost, Fig. 5 View Figures 1–6 ), as well as bases of other segments; dark portions of antennal segments covered with very fine sparse darkbrown pubescence ( Fig. 6 View Figures 1–6 ).
Prothorax in males and females about as long as basal width (or about 1.1 times shorter than basal width); anteriorly thorax wider than posteriorly; lateral spines long and acute, slightly located in front of middle; pronotum convex; most of pronotal area glabrous, roughly sculptured, with small irregular punctation and often with small scattered white spots; each dot bears a small short pale seta; central elongated area more or less smooth, irregular, surrounded by white curved lines often partly reduced, which can be accompanied with bigger elongated white spots (in the most pubescent female), sometimes pronotum without any white spots (in the least pubescent male).
Scutellum triangular, with glabrous wide or narrow area along middle and dense white pubescence laterally. Elytra moderately wide, widest near middle, or sometimes before middle, in males about 2.1 times longer than middle width, in females 1.9–2.0 times longer than middle width; strongly attenuated posteriorly in males, or moderately attenuated in females; humeral angles distinct, but rounded; elytral punctation large, dense, irregular, partly joined; dorsal elytral carinae hardly visible or totally obliterated; humeral carinae obliterated, smooth, with about same punctation as dorsal elytral surface; sutural white stripe very narrow, often partly or totally lost; humeral and external dorsal stripes strongly reduced; in males only apical parts of stripes developed; in females external dorsal stripes nearly complete, though irregular and many times interrupted anteriorly, or represented in anterior half by a row of spots only; humeral stripes in females never reaching elytral anterior half; sometimes in females internal dorsal elytral stripes also presented as a row of small white spots anteriorly; marginal stripes usually complete, but in males strongly narrowed anteriorly or totally disappearing in elytral anterior half (holotype); in females marginal stripes rather wide along elytral posterior half, became narrow anteriorly and here partly reduced or several times interrupted.
Legs long and thin; hind tibiae in males less than 2.0 times of length of hind tarsi; hind tibiae in females more than 2.0 times of length of hind tarsi; 2nd and 3rd tarsal segments subequal in length; 1st segment of hind tarsus about 2.0 times longer than 2nd or 3rd segments; 3rd tarsal segment emarginated near middle or a little deeper; claw segments of anterior tarsi in males a little shorter than 2nd and 3rd segments combined; claw segments of anterior tarsi in females longer than 2nd and 3rd segments combined; femora nearly pubescent externally, especially in females; femora in males usually partly glabrous externally; internal sides of middle and posterior femora nearly glabrous, internal sides of anterior femora partly pubescent; all tibiae and tarsi covered with very dense white pubescence.
Abdomen covered with dense white recumbent pubescence, with scattered small glabrous spots in males, less numerous in females; pygidium in males widely rounded, postpygidium and last abdominal sternite shallowly emarginated; last abdominal tergite narrowly rounded and last abdominal sternite narrowly truncated in females.
Material examined. Holotype male, Inner Mongolia, Alxa Left Banner, Tengger Desert , elev. 1314 m, 38°28′N, 105°13′E, host plant: Psammochloa villosa (Trin.) Bor , 3.X.2018, leg. Xuefeng Chen ( IZCAS, IOZ (E) 2002909). Paratypes (8 specimens). 2 males, 2 females, same data as holotype (one male and one female in IZCAS, IOZ (E) 2002910 and IOZ (E) 2002911, another two in CMD); 1 male, 1 female, same data to holotype but 2.X.2018 ( CCXF) GoogleMaps ; 1 female, Inner Mongolia, Alxa Left Banner, Tengger Desert , elev. 1314 m, 38°28′N, 105°13′E, host plant: Psammochloa villosa (Trin.) Bor , 13.VIII.2018, leg. Jianhu Shen ( IZCAS, IOZ (E) 2002912); 1 male, Inner Mongolia, Alxa Left Banner, Tengger Desert, elev. 1312 m, 38°31′N, 105°01′E, 1.X.2017, leg. Xinxin Zhao ( IZCAS, IOZ (E) 2002913) GoogleMaps .
Host Plant. Psammochloa villosa (Trin.) Bor ( Figs 7–8 View Figures 7–10 ).
Biology. Adults are active in the daytime in the sandy deserts ( Figs 9–10 View Figures 7–10 ) from the middle of August to the beginning of October; the first specimen collected on 1.X.2017, was found at 10: 00 am, while those collected on 3.X.2018 were found during 9:30–15:00 eating leaves and stems of Psammochloa villosa (Trin.) Bor. Oviposition takes place on bases of the stems of host plants. Beetles try to escape by quick crawling (cannot fly for hind wings reduced). One individual was videoed on 19.VIII.2018, at the locality which is beside the Tian’ehu (Swan lake) of Alxa Left Banner, Tengger Desert (personal communication with Li Ren and Bao Li, video taken by Jun Du), showing that it can crawl quite fast on the sand.
Distribution. China (Inner Mongolia).
Etymology. The species is named after Mr. Xinxin Zhao (Inner Mongolia, China), who collected the first specimen of the type series and kindly donated it to IZCAS.
| IZCAS |
Institute of Zoology, Chinese Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.