Nandeva Wiedenbrug, Reiss et Fittkau
|
publication ID |
https://doi.org/ 10.5281/zenodo.204580 |
|
DOI |
https://doi.org/10.5281/zenodo.6194517 |
|
persistent identifier |
https://treatment.plazi.org/id/122B9B0D-0424-0E57-FF2B-BCE6FA419F31 |
|
treatment provided by |
Plazi |
|
scientific name |
Nandeva Wiedenbrug, Reiss et Fittkau |
| status |
|
Nandeva Wiedenbrug, Reiss et Fittkau View in CoL View at ENA
Nandeva Wiedenbrug, Reiss et Fittkau, 1998: 59 View in CoL
Type species. Nandeva gaucha Wiedenbrug, Reiss et Fittkau, 1998: 60 , by original designation; Brazil [male, pupa].
Other included species:
Nandeva chilena Wiedenbrug, Reiss et Fittkau 1998: 65 View in CoL ; Chile [pupa].
Nandeva digitifer View in CoL sp. n.; Chile [male, female].
Nandeva fittkaui Cranston, 1999: 296 View in CoL ; Australia [male, female, pupa].
Nandeva latiloba Saether et Roque, 2004: 65 View in CoL ; Brazil, Venezuela [male, female].
Nandeva strixinorum Saether et Roque, 2004: 67 View in CoL ; Brazil, Mexico [male].
Nandeva tropica Wiedenbrug, Reiss et Fittkau, 1998: 64 View in CoL ; Brazil, Panama, Venezuela [pupa; male ( Saether & Roque 2004: 68)].
Nandeva verruculata View in CoL sp. n.; Brazil [male].
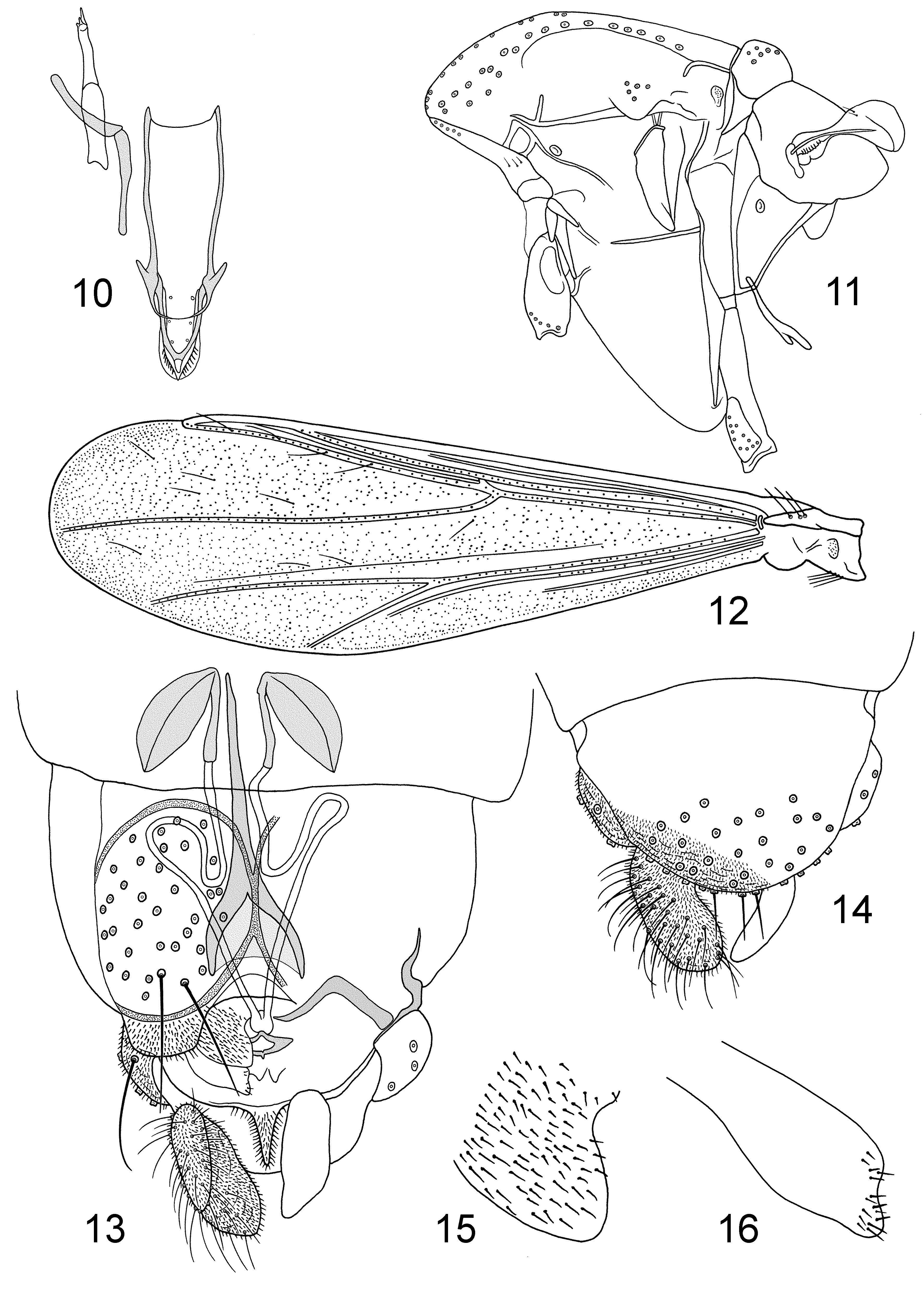
Diagnostic characters. The males are separable from other Tanytarsini View in CoL by having bare eyes with no dorsomedian elongation, dorsal antepronotal setae, costa ending proximal to distal end of M3+4, subcosta and anal vein normally without setae, 0–4 setae on squama, tibial combs all with spurs, anal point long and parallel-sided or spatulate, median volsella absent, superior volsella at most with a few basal microtrichia, sometimes with apparent digitus. From Chironomini View in CoL the males differ by having RM oblique to R4+5 at least in the Neotropical species, and a combination of antenna with 13 flagellomeres, fore tibial scale with long spur, 0–4 setae on squama, and tergite VIII anteriorly tapered. The females have gonapophysis VIII well divided with straight ventrolateral lobe and short notum. In the Neotropical species the ventrolateral lobe is dorsal to the dorsomesal lobe and with only apical microtrichia conspicuous, the spermathecal ducts has loops or bends, a floor is distinct, and the setae of tergite IX are not divided into two groups. The Australian N. fittkaui View in CoL has the ventrolateral lobe in the same plane as the dorsomesal lobe and covered by strong microtrichia, the spermathecal ducts are nearly straight, a floor apparently is absent, and the setae of tergite IX are divided into two groups. The pupa differs from other Chironominae View in CoL by lacking thoracic horn, frontal setae, anal lobe fringe, anal spur or comb and pedes spurii A and B, and by having paired or fused anterior spine patches on tergites III–VII or III–V and posterior hook rows on tergites II–V or VI.
Description. Imagines. Small species, wing length 0.7–1.2 mm. Coloration pale to dark brown, abdomen usually banded. Eyes without dorsomedian elongation, bare. Male antenna with 13 flagellomeres, fully plumed. Antennal ratio of male 0.2–0.8. Female antenna with 5 flagellomeres, flagellomeres 2–4 flask-shaped. Antennal ratio of female 0.1–0.2. Temporals uniserial, consisting of 4–13 inner verticals and / or frontals, 1–4 outer verticals and 2–4 postorbitals. Clypeus with 6–19 setae. Five long palpomeres, third palpomere with 2–4, lanceolate or scalpellate sensilla chaetica, fourth palpomere shorter than third.
Antepronotal lobes medially reduced, with 2–6 dorsal, 0–4 median, and 2–8 lateral setae. Acrostichals 10–28; dorsocentrals 12–25, partly biserial; prealars 3–7; supraalar absent. Scutellum with 6–12 uniserial setae.
Wing cuneiform. Venation of Tanytarsini type with RM continuous with R4+5 and with R4+5 ending proximal to M3+4 (not known for N. fittkaui ); membrane clear, with setae in all cells and on all veins except subcosta and anal vein usually bare. VR 1.18–1.39. Costa not extended, postcubitus long with anal vein shorter. Brachiolum with 2–5 setae, subcosta with setae in some N. strixinorum only, R with 13–40 setae, R1 with 6–34, R4+5 with 18–52, M with 0–35, M1+2 with 20–53, M3+4 with 13–50, Cu with 14–53, Cu1 with 8–32, postcubitus with 13–59 setae. Squama bare in N. fittkaui , with 1–4 setae in Neotropical species.
Tibial combs all with spurs; anterior tibia with long, thin slightly curved spur (not known for N. fittkaui ); tibial combs separate. Leg ratio of male 0.83–1.18. Tarsi with beards on all legs. Pulvilli absent.
Both tergites and sternites with basal, marginal and lateral setae; on sternites basal and marginal setae reduced to anterior and posterior clusters ( Wiedenbrug et al. 1998: fig. 2A, B). Tergite VIII anteriorly tapered.
Tergal bands of widely separated V-type. Tergite IX of male with 12–22 setae at base of anal point, laterosternite IX with 3–8 setae. Anal point 33–69 µm long, parallel-sided to slightly tapering or, in N. fittkaui , spatulate, free of microtrichia. Transverse sternapodeme straight, no oral projections. Phallapodeme 47–85 µm long. Gonocoxite normally 0.7–1.3 times as long as gonostylus, sometimes extending beyond attachment point of gonostylus. Superior volsella 21–57 µm long; with broadened base and narrow, curved extension, extension 3–14 µm wide; bare or at most with a few microtrichia in basal two-thirds; with 1–3 apical setae; inner margin with 0–1 median and 0–1 basal setae; outer margin with 1–3 basal setae, sometimes on tubercle; digitus sometimes present, with 1 apical seta. Median volsella absent. Inferior volsella 38–73 µm long, with 6–14 apical to median setae, 0–2 basal setae and long microtrichia. Gonostylus slender, 95–190 µm long.
Gonocoxapodemes VIII forming well sclerotized complete circles, each encircling 23–33 setae. Floor developed except in the Australian species. Tergite IX of female rounded or somewhat triangular with rounded apex, either with two groups each of about 8–9 setae ( N. fittkaui ) or 17–26 un-grouped setae. Gonocoxite weak to well developed, with about 5 setae. Gonapophysis VIII divided into triangular dorsomesal lobe and straight ventrolateral lobe. Ventrolateral lobe in Neotropical species placed dorsal of dorsomesal lobe, without microtrichia except for a few distinct apical microtrichia; in N. fittkaui ventrolateral lobe in the same plane as the dorsomesal lobe and covered with strong microtrichia. Apodeme lobe indistinct to distinct. Postgenital plate sharply triangular. Cerci of moderate size, about as long as the short notum. Seminal capsules usually ovoid to circular; spermathecal ducts with loop, strong bend or, in N. fittkaui straight, without bulbs before common opening.
Pupa. As in Wiedenbrug et al. (1998) and Cranston (1999).
Remarks. The Australian N. fittkaui differs from the Neotropical species in several aspects. In the male there are no lateral antepronotals, the squama is bare, the anal point spatulate and the superior volsella carries more basal setae and strong microtrichia. In the female the gonocoxapodeme is not visible, the coxosternapodeme faint, the gonocoxite IX not developed and tergite IX small with setae divided into lateral groups. In the pupa the anteromedian patches of stronger spinules are lacking on tergites VI–VII. All this may add up to that Nandeva fittkaui should be placed in a different genus. However, both the male and the female were described on pharate specimens and the lack of sclerotization of the gonocoxapodeme and the coxosternapodeme in the female as well as some other features such as the lack of lateral antepronotals may be artifacts.
The gonapophysis VIII of the Neotropical species appears unique among chironomids in having the ventral lobe situated dorsal of the dorsomesal lobe and being without microtrichia except at apex. A similar ventrolateral lobe has been described only from Harnischia curtilamellata Malloch ( Saether 1977 fig. 89 A, B).
Distribution. The genus previously was known from Brazil, Chile, Panama and Australia. The present paper gives additional records from Mexico and Venezuela.
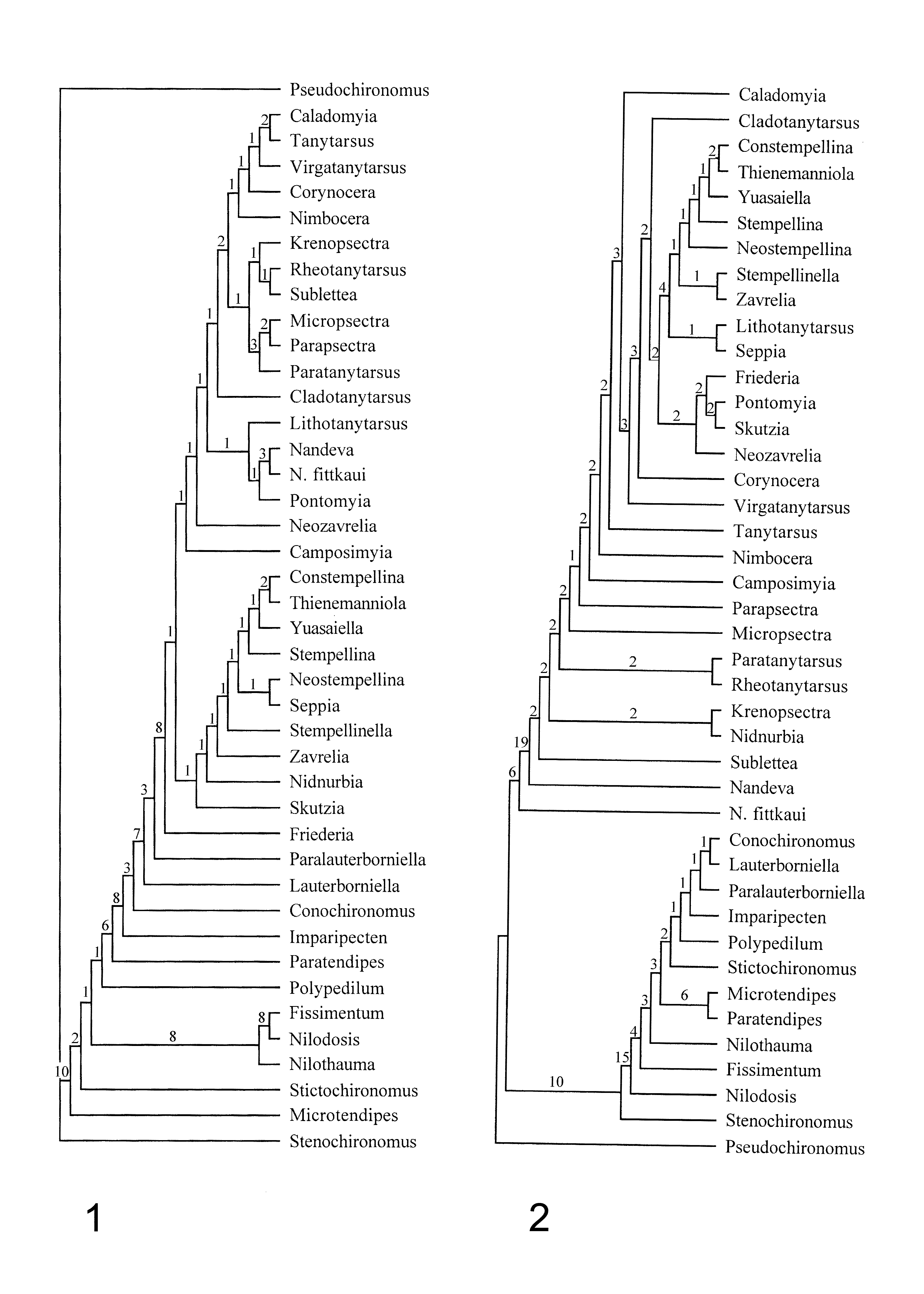
Systematics. The parsimony analysis in Saether & Roque (2004) had Nandeva either as the sister genus of all the Tanytarsini ( Saether & Roque 2004, fig.13), only Friederia Saether et Andersen was more basal in the cladogram ( Saether & Roque 2004, fig.14), or near the base of the Tanytarsina ( Saether & Roque 2004, fig.12). The differences between the Australian N. fittkaui and the Neotropical species indicate that the former should be placed in a separate genus and N. fittkaui thus is entered separately in the data matrix.
Nandeva fittkaui has a relatively broad, somewhat spatulate anal point. A third character alternative (spatulate) thus is added to character 28.
An additional character for the female tergite IX is added: (0) setae not divided into two groups; (1) divided into two groups. Amongst the taxa included in the data matrix only N. fittkaui and Lauterborniella Thienemann et Bause show character alternative 1.
The new species described here show the presence of a possible digitus in N. digitifer and perhaps in N. verruculata . The character state of character 32 thus should be changed from 1 to 0&1. A floor is present in the Neotropical species, but not in N. fittkaui . Trend 47 thus is scored 0 for N. fittkaui , 1 for the other Nandeva species. Saether (1977:142, fig. 63 D) found a large floor in female Zavrelia Kieffer. However, Ekrem & Stur (2009) reviewed the genus Zavrelia and none of the reviewed and described species presented a vaginal floor. Ekrem & Stur (2009) pointed out that until evidence is presented that associated Zavrelia females possess a floor, the lack of a vaginal floor should be diagnostic to Zavrelia . Trend 47 for Zavrelia thus is changed from 0 to 1.
Some characters both in Saether and Roque (2004) and in the present analysis are scored with one character alternative even if they really are polymorphic. Character 75 in Saether and Roque (2004) for instance is for Micropsectra scored as if all species had a spur on the antennal pedestal although a few species lack this spur. The lack of a spur almost certainly is secondary and scoring 0&1 will make the trend less informative.
Alternative 0 for character 80 in Saether and Roque (2004) should be changed to “always simple” as the other alternatives also includes “simple” and really are underlying synapomorphies scored as synapomorphies.
Running the parsimony analysis with these character states altered results in a few changes. Without weighting and after reweighting as in Fig. 12 View FIGURES 10 – 16 in Saether and Roque (2004) the Tanytarsini is divided into two subtribes with Friederia as sister group to the other Tanytarsini ( Fig. 1 View FIGURES 1 – 2 ). Among other changes are that Nidnurbia Säwedal and Skutzia Reiss are included in the Zavreliina. With some characters weighted and reweighted as in Saether and Roque (2004 fig. 14) Nandeva fittkaui is the sister to other Nandeva plus the remaining Tanytarsini . Zavreliina is monophyletic while the Tanytarsina is not ( Fig. 2 View FIGURES 1 – 2 ). The Bremer support is relatively high for Tanytarsini as a monophyletic tribe in Figure 1 View FIGURES 1 – 2 , for Tanytarsini without N. fittkaui in Figure 2 View FIGURES 1 – 2 .
Nandeva fittkaui differs significantly from the Neotropical species as male, female and pupa. Particularly the female indicates that the species should be placed in a separate genus, and if the wing venation not is of the Tanytarsini type, in a different tribe. However, the result from the parsimony analyses indicates that the species could be closely related to the Neotropical species. The erection of a new genus for N. fittkaui should wait for a description of the wing venation and of the tibial spurs.
After the completion of this paper three papers relevant to the analysis have been published. Ekrem et al. (2010) suggest that Krenopsectra Reiss and Parapsectra Reiss should be included in Micropsectra and Sanseverino et al. (2010) synonymize Nimbocera Reiss with Tanytarsus . Although we accept these synonyms we have kept the taxa separate in order to check if they are confirmed by our findings. While the inclusion of Parapsectra in Micropsectra is confirmed and Nimbocera in Tanytarsus possible, the synonymy of Krenopsectra with Micropsectra appears more doubtful.
The third paper, a dated molecular phylogeny for the Chironomidae, Cranston et al. (2011) suggests that Nandeva should be included in the tribe Pseudochironomini . Figure 2 View FIGURES 1 – 2 suggests that at least N. fittkaui could belong in Pseudochironomini . Making Pseudochironomus Malloch plus Nandeva a monophyletic sister of Tanytarsini in Fig. 2 View FIGURES 1 – 2 will when examined in McClade in fact make the tree one step shorter.
However, several critical taxa of Pseudochironomini and other basal Chironominae remain unsampled and may lead to significantly different results. Prior to the inclusion of Buchonomyia Brundin and Shangomyia Saether et Wang in the molecular phylogeny Cranston et. al. (2000) found: “The major robust finding at subfamilial level is confirmation from both molecules under all constraints of Saether's postulation of the basal position of the subfamily Telmatogetoninae , as sister to the remaining subfamilies, analyzed with maximum parsimony” and that Xiaomyia Saether et Wang was sister to Corynoneura van der Wulp.
The disagreement between Brundin and Saether (1978), Saether (1989, 2000) and Murray and Ashe (1981, 1985) mainly consists in the importance of presence or absence of a larval premandible as an external sclerite. It was agreed that the premandible had to have been present in the chironomidal ancestor, but while Murray and Ashe thought that the reduction was so important that it could have taken place only once, Brundin and Saether argued that the weight of other characters made it more likely that the reduction had taken place twice and was an underlying synapomorphy. According to the result of the molecular phylogeny of Cranston et al. (2011) the reduction must have taken place three times, i.e. confirming the reduction as an underlying synapomorphy in accordance with Brundin and Saether. However, the most parsimonious explanation is that the premandible not is homologous with the premandible of other Diptera . and thus an objective synapomorphy for the Telmatogetoninae plus the Chironomoinae.
The results of Cranston et al. (2011) show that there are exceptions to nearly all “objective” synapomorphies in all subfamilies and that different groups mostly are held together by a web of underlying synapomorphies. The larvae of Nandeva are known, but undescribed. A future analysis should include the larva and have Xiaomya and
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Chironominae |
Nandeva Wiedenbrug, Reiss et Fittkau
| Andersen, Trond, Saether, Ole A. & Contreras-Ramos, Atilano 2011 |
Nandeva latiloba Saether et Roque, 2004 : 65
| Saether 2004: 65 |
Nandeva strixinorum Saether et Roque, 2004 : 67
| Saether 2004: 67 |
Nandeva fittkaui
| Cranston 1999: 296 |
Nandeva Wiedenbrug, Reiss et Fittkau, 1998 : 59
| Wiedenbrug 1998: 59 |
Nandeva chilena Wiedenbrug, Reiss et Fittkau 1998 : 65
| Wiedenbrug 1998: 65 |
Nandeva tropica Wiedenbrug, Reiss et Fittkau, 1998 : 64
| Saether 2004: 68 |
| Wiedenbrug 1998: 64 |