Treptacantha rayssiae (Ramon) M.Mulas, J.Neiva
|
publication ID |
https://doi.org/ 10.5252/cryptogamie-algologie2020v41a10 |
|
DOI |
https://doi.org/10.5281/zenodo.7828089 |
|
persistent identifier |
https://treatment.plazi.org/id/115587B0-0802-FFAB-FF63-817CFB26F96A |
|
treatment provided by |
Felipe |
|
scientific name |
Treptacantha rayssiae (Ramon) M.Mulas, J.Neiva |
| status |
comb. nov. |
Treptacantha rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov.
Cystoseira rayssiae Ramon , Israel Journal of Plant Sciences 48: 59 (English), 61 (Latin); figs 1-5 (fig. 1: holotype) (1970) (basionym).
TYPE MATERIAL. — Holotype. HUJ ( Ashqelon , Israel; 23.V. 1953).
DESCRIPTION
The morphological characteristics of this species along the Israeli coast are well in accordance with the features of the genus as described by Orellana et al. (2019), although Ramon herself noted that the species exhibits considerable morphological plasticity ( Figs 3 View FIG ; 4 View FIG ). Treptacantha rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., shows a tophulose, non-caespitose habit, growing up to c. 30 cm high ( Figs 3A View FIG ; 4A View FIG ). The thallus is attached to the substrate by a basal disc from which a cylindrical simple or branched axis grows ( Figs 3D View FIG ; 4C View FIG ). Tophules are present (albeit in young thalli they are poorly distinguishable) and can be of different shapes (from obovate, ovate, to club-shaped, spherical and oblong), are 3-8 mm long and up to 4 mm broad ( Figs 3B View FIG ; 4B View FIG ), and often concentrated in the tip of the main axes. Holdfast, main axes, and tophules are perennial. Primary branches are seasonal (March-June) ( Mulas et al. 2019), typically smooth in the basal region and occasionally with small and widely spaced spiny appendages. Branches of higher order are more robust, show lateral spines, and their inner aerocysts are inconspicuous.Transformed ends of last order branches show spiny laterals containing receptacles ( Fig.3C View FIG ). Differences in the morphology among specimens seem to be environmentally driven. For instance, subtidal specimens display shorter and robust branches densely covered with spinelike appendages and without pronounced receptacles compared to tide pool specimens that have less pronounced spines and correspond to Ramon’s description ( Fig. 4 View FIG ).
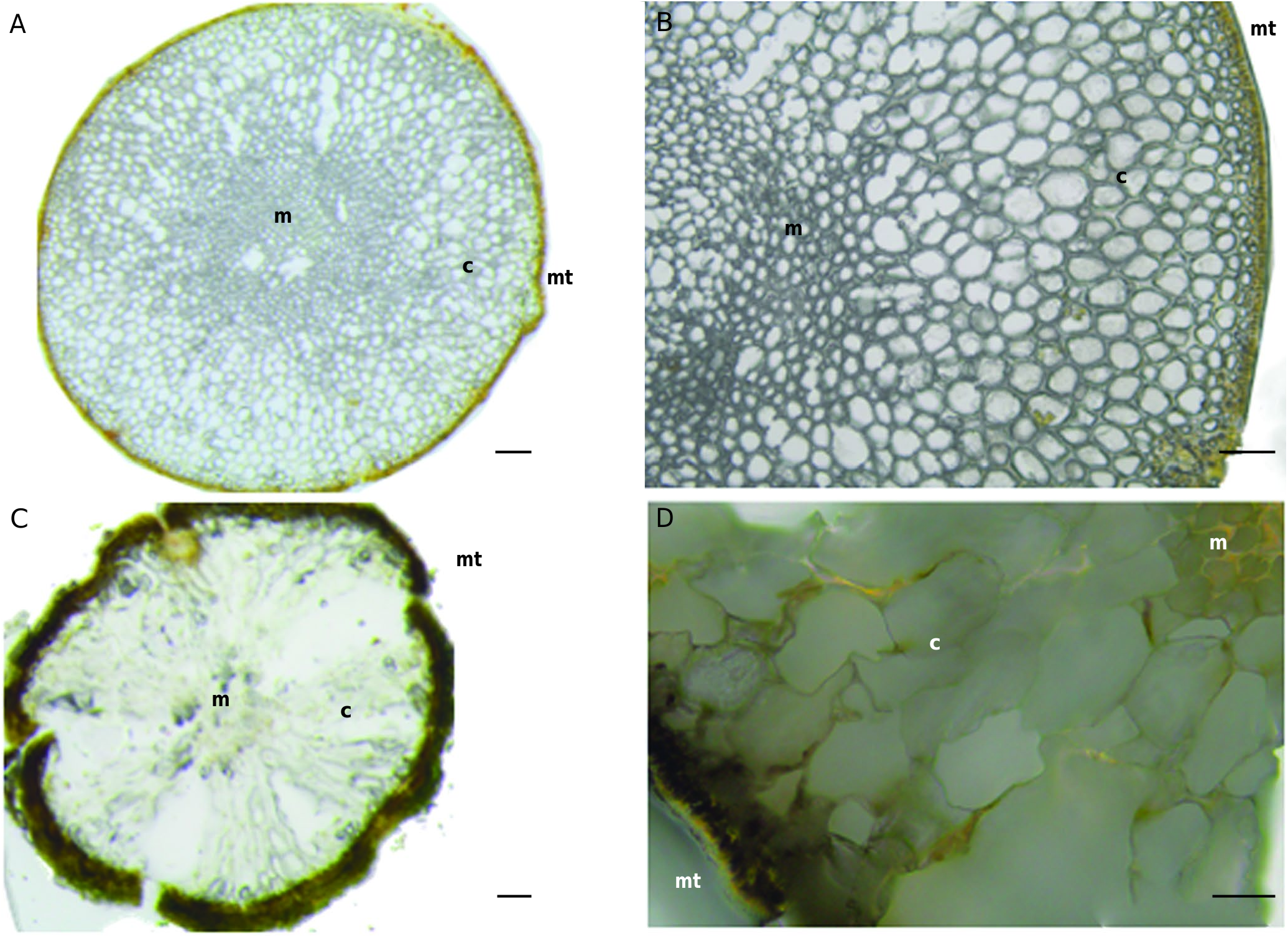
Anatomical characteristics of T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., cross sections corresponded to the genus Treptacantha as recently described by Orellana et al. (2019). T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., shows medullary cells which form a central mass, while the cortical ones having a bigger size, globose shape and thickened walls and the meristoderm is composed by a single layer of square-shaped cells ( Fig. 5 View FIG ).
This is in accordance with the description of the genus ( Orellana et al. 2019): Treptacantha often displays significant polymorphism attributable to regional, seasonal and habitat differences (e.g. genetically – confirmed Atlantic Treptacantha nodicaulis , Treptacantha sp. from Crete, T.baccata , T.barbata , T.abies-marina , T. ballesterosii and T. mauritanica ) stressing that morphological differences must be supported by additional evidence, such as molecular data, before describing additional species.
Species distribution
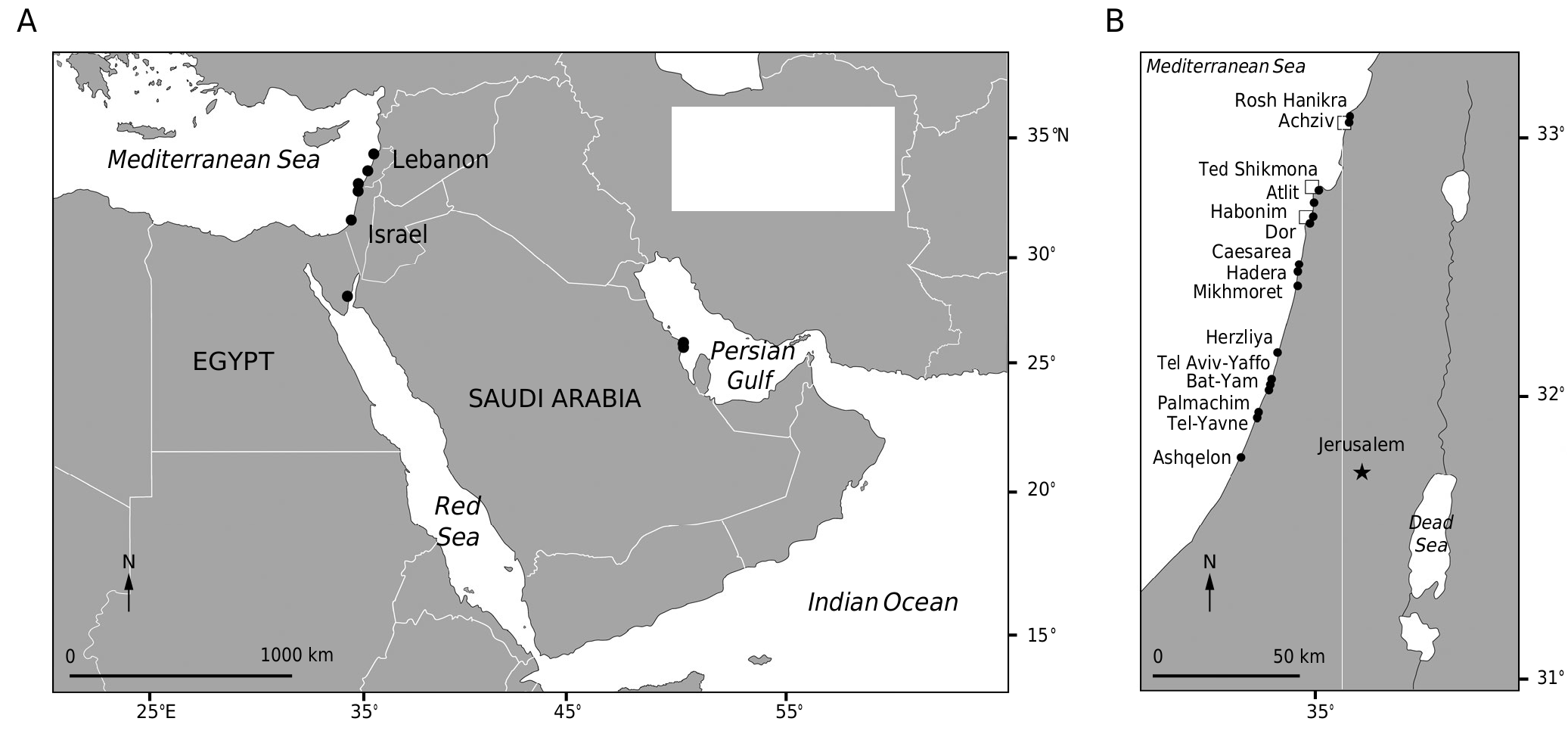
Literature and database records suggest a disjunct geographical distribution for T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., as shown in Figure 1A View FIG . Treptacantha rayssiae can be found in tide pools associated with vermetid reefs (abrasion platforms) in north Israel ( Rilov et al. 2020), and as scattered individuals (in several locations), or as an extensive forest (only in one location, in Haifa) on horizontal subtidal bedrocks down to 5 m depth ( Fig. 1B View FIG , based on Ramon 2000, Mulas et al. 2019, Peleg et al. 2019 and personal observations). In addition to Israel ( Ramon 2000; Einav & Israel 2008), T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., has been also recently reported from several sites in Lebanon (Nakoura, Adloun, Barbara, Ras-Chekaa) ( Badreddine et al. 2018). Surprisingly, extra-Mediterranean records were also reported from one site (Dahab) in the Red Sea along the Egyptian coast of the Gulf of Aqaba (Abdel-Raouf et al. 2015) and from six sites in the Persian Gulf (Ras Tanura, Saftwah, Al Qatif, Sayhat, Ad Dammam and Al Azizayah) in Saudi Arabia (Abdel-Kareem 2009).
These extra-Mediterranean records have not been genetically confirmed. In case they are not misidentifications, three exclusive scenarios can be postulated. In the first scenario, T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., may be a palaeoendemic species that was formerly widespread (before the closure of the Mediterranean passages to the Indian Ocean, some 20 MYA), and is now restricted to several very small “relict” areas of its past distribution. In the second scenario, T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., may be a Lessepsian migrant first detected in the invaded region, the Levantine basin, and later in the origin region, the Red Sea and the Persian Gulf. These two scenarios seem unlikely because T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., and T. nodicaulis are more closely related than T. nodicaulis and T. baccata , and the two latter are estimated to have diverged around 10 MYA ( Silberfeld et al. 2010), i.e., already after the closure of the eastern Tethyan seaway ( Bialik et al. 2019). Under the third scenario, T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., is an eastern Mediterranean endemic seaweed that has migrated to the Indo-Pacific, a rare case of anti-Lessepsian migration. It should be noted that there are only a handful examples of anti-Lessepsian species, making this last scenario also unlikely ( Golani et al. 2002). Among the very few marine organisms that have moved from the Mediterranean into the Red Sea, are the fish Solea aegyptiaca Chabanaud , and six species of polychaetes ( Golani et al. 2002; Faiza 2009; Chanet et al. 2012), but no macroalgal species recorded so far. In contrast, a large number of Lessepsian macroalgae migrants have been recorded in the Mediterranean Sea along the years ( Por 1971, 1978; Galil & Zenetos 2002; Rilov & Galil 2009; Otero et al. 2013; Boudouresque et al. 2016; Galil et al. 2017; Israel & Einav 2017), where the last update has counted 119 alien macrophytes introduced by different sources out of a total of 613 confirmed marine organisms ( Verlaque et al. 2015; Zenetos et al. 2017). All possible hypothetical scenarios to explain the extra-Mediterranean records are not supported by evidence. The main growing/reproductive season of T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., is winter- spring and not warmer summer months when the fronds are shed, and the basal perennial parts enter a dormancy period ( Mulas et al. 2019). A temperate growth and reproductive window do not support a tropical origin, but seasonal shifts are also observed among tropical species. Because all three scenarios are highly unlikely, we suspect that the records from the Persian Gulf and the Red Sea (Abdel-Kareem 2009; Abdel- Raouf et al. 2015) are based on misidentifications. In the study of Abdel-Kareem (2009), the photographic record has poor quality but does not resemble T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov. In fact, in both studies of Abdel-Kareem (2009) and Abdel-Raouf et al. (2015), the taxonomical identification of T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov. (as Cystoseira rayssiae ) was based on the reference check-list of the Red Sea of Lipkin & Silva (2002) which, however, never mentions T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., but rather the common fucoid Polycladia myrica (as Cystoseira myrica ). This species and Syrophysalis trinodis (as Cystoseira trinodis ) have also been reported from the Red Sea in other studies (e.g. Ibraheem et al. 2014). Many seaweed groups are notoriously difficult to identify, and this applies also to Cystoseira sensu lato and related genera. Thus, we conclude that the most plausible explanation is that T. rayssiae (Ramon) M.Mulas, J.Neiva & Á. Israel, comb. nov., was misidentified in these studies, and the species is a unique example of a Levantine Basin endemism. If what we claim is confirmed by further analyses of Mediterranean samples, conclusively excluding it from nearby areas ( Cyprus, southern Turkey and farther away), the protection of this species emerges as a priority in the Mediterranean Sea, because of the restricted local distribution and increasing pressures such as rabbitfish and sea urchin overgrazing, pollution, ocean warming and urbanization.
| HUJ |
Hebrew University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |