Trichopria anastrephae Lima, 1940
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4858.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:A2E85BBC-F1DA-41FE-B2A2-AA086F39186E |
|
DOI |
https://doi.org/10.5281/zenodo.4504688 |
|
persistent identifier |
https://treatment.plazi.org/id/1137956E-FFB8-FFF3-FF27-B73EFEF1FC59 |
|
treatment provided by |
Plazi |
|
scientific name |
Trichopria anastrephae Lima, 1940 |
| status |
|
Trichopria anastrephae Lima, 1940
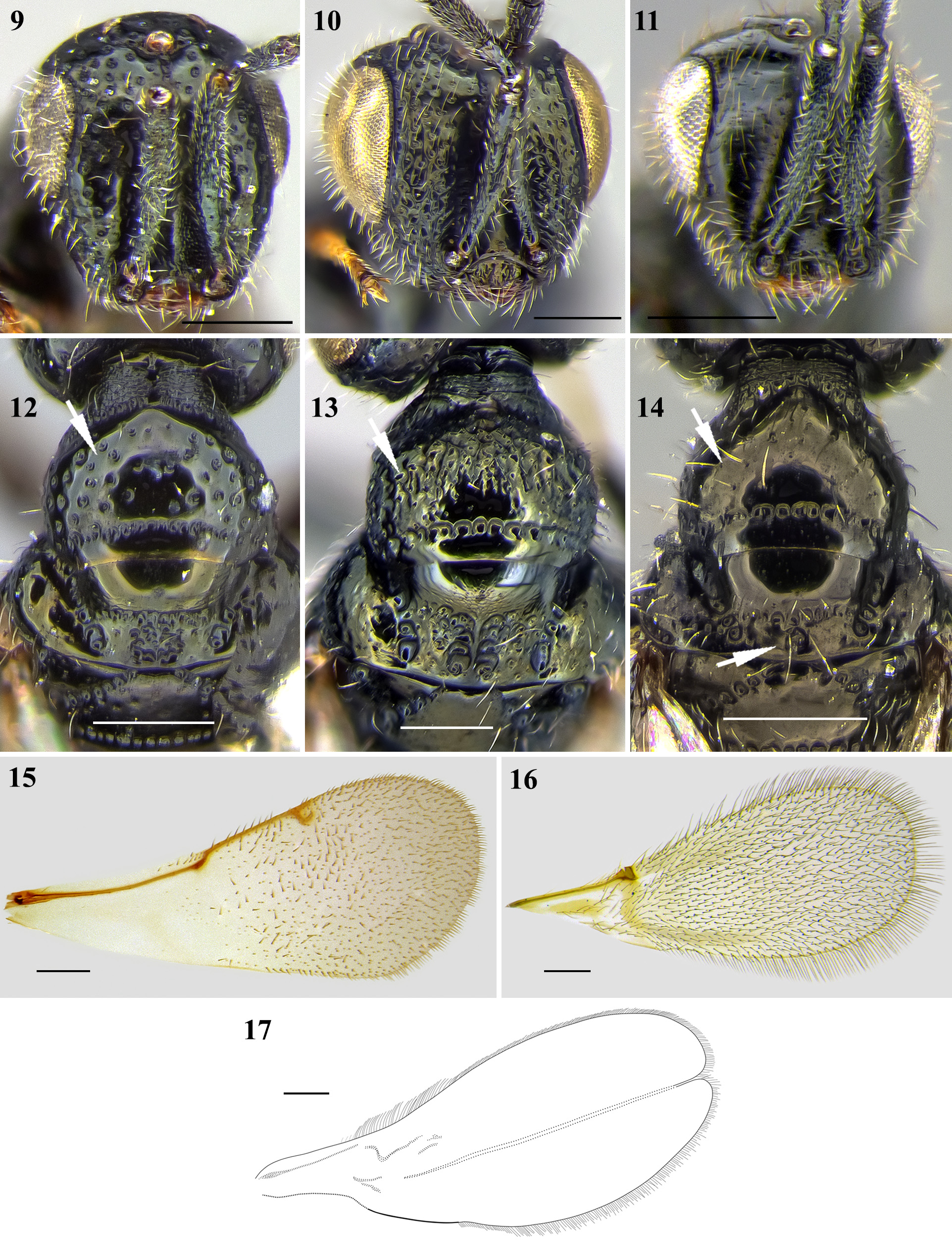
( Figs 1, 2 View FIGURES 1–8 , 16 View FIGURES 9–17 )
Diagnosis. Body dark-brown to black, surface mostly smooth and polished except petiole. Fore wing fully developed, without closed cells; with complete, short, subcostal vein (=submarginal vein), ending in a short marginal vein (genus Trichopria ). Female antennae 12-segmented, with 3-segmented clava; male antennae 14-segmented; flagellomeres long and pedunculate, swollen apically with long setae. Scutellum smooth, median carina absent; scutellar sulcus smooth and shallow. Body length 1.8–2.0 mm. Considering the Neotropical fauna, Tr. anastrephae is similar to Trichopria peraffinis (Ashmead) , which is a much smaller species (~1.0 mm long), and presents a small pit on scutellum anteriorly (absent in Tr. anastrephae ).
Taxonomy. Trichopria is one of the largest genera in Diapriidae , and no revision including the Neotropical fauna has been published, making identification to species level difficult. For instance, there are at least two unidentified species of Trichopria that parasitize species of Anastrepha in the New World ( USA and Costa Rica) ( Ovruski et al. 2000). Of the 12 species of Trichopria in Brazil ( Margaría 2020), nine can be keyed out using Kieffer’s (1910; 1916) identification keys in combination with original descriptions ( Fouts 1926) or recent diagnosis ( Notton 2014). Trichopria catarinensis Ferrière is discarded because of its specialized biology, as parasitoid on Ecitonini ( Hymenoptera , Formicidae ). The remaining species, Trichopria lamellifera Ogloblin could also be discarded based on host association with Micropezidae ( Diptera, Nerioidea ), and morphological differences such as the length of antennomeres and its setae in males being much longer in T. anastrephae (antennomere 4 ~ 200 µm and longest setae ~ 300 µm) than in T. lamellifera (91 µm and 98 µm respectively), and females with much larger compound eyes (eye diameter ~5x longer than malar space in T. anastrephae compared to ~2.5x in T. lamellifera ) ( Ogloblin 1934). Trichopria is a diverse genus, with possibly a high number of undescribed species in the neotropics (Masner & García 2002). Therefore, caution is advised when using the identification key presented below. A revision of the genus is badly needed, for the Neotropical region.
Biology. Trichopria anastrephae is an endoparasitoid koinobiont on pupae of Tephritidae and less frequently on Drosophilidae , for example Drosophila suzukii (Matsumura) (Yoder 2007) . Known Tephritidae hosts are A. serpentina and A. fraterculus in Brazil ( Lima 1940 and Aguiar-Menezes et al. 2001, respectively) and Ce. capitata in Argentina ( Turica & Mallo 1961).
Biological control. The potential of Tr. anastrephae as a biological agent has not been investigated in detail, although it is likely to be an important natural enemy of tephritids in Brazil, being the most common parasitoid species in star fruits ( Silva et al. 2003).
Distribution. Brazil and Argentina.
Distribution in Brazil (associated with tephritid species). BA ( Souza-Filho et al. 2007), CE ( Silva et al. 2020), GO ( Marchiori & Penteado-Dias 2001), MG ( Silva et al. 2003), RJ ( Lima 1940), SC ( Garcia & Corseuil 2004), RS ( Cruz et al. 2011).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |