Rhadinorhynchus hiansi Soota and Bhattacharya, 1981
|
publication ID |
https://doi.org/ 10.1645/19-97 |
|
DOI |
https://doi.org/10.5281/zenodo.7753019 |
|
persistent identifier |
https://treatment.plazi.org/id/0F7987C5-477A-2344-65C0-FE23FEEF11ED |
|
treatment provided by |
Felipe |
|
scientific name |
Rhadinorhynchus hiansi Soota and Bhattacharya, 1981 |
| status |
|
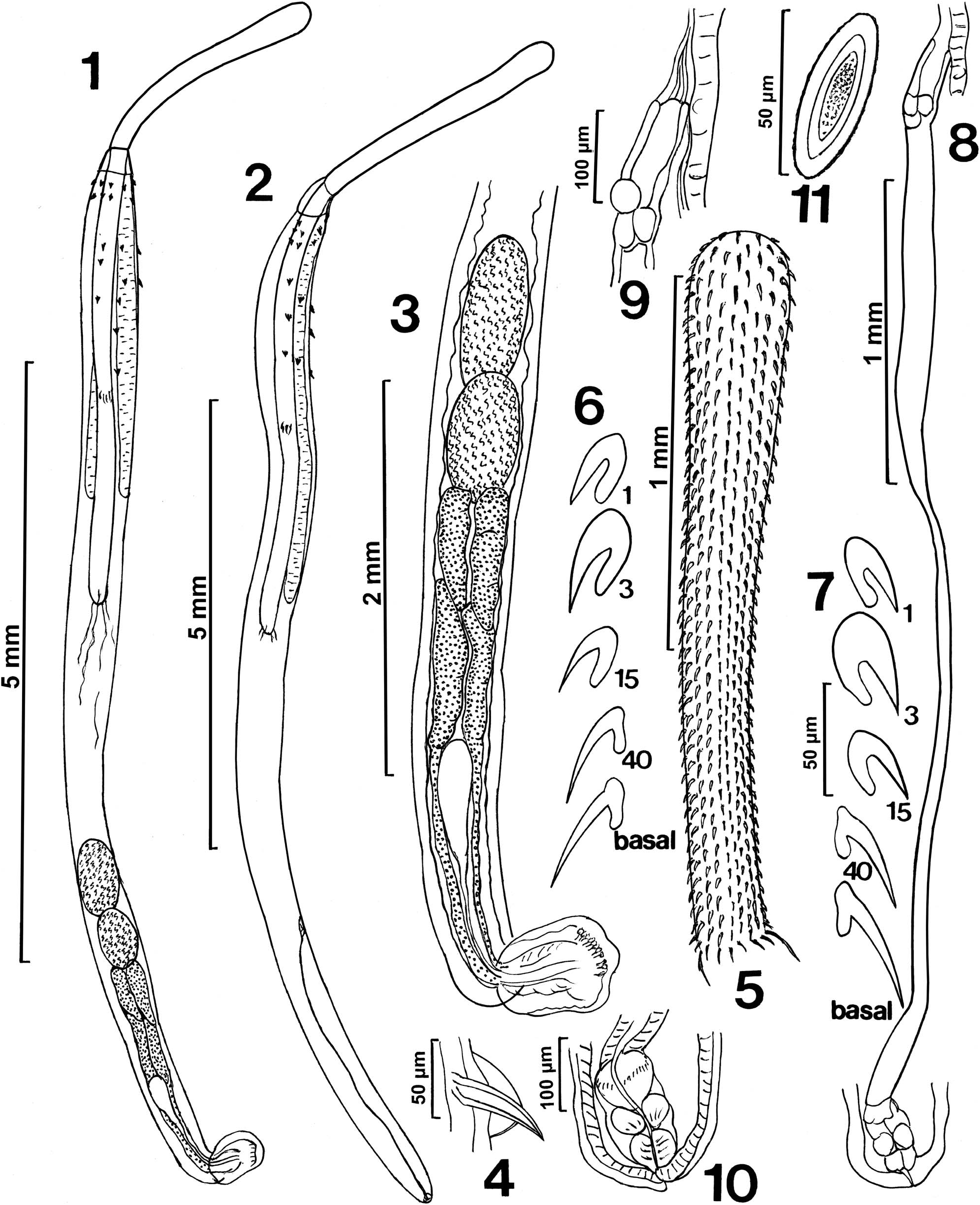
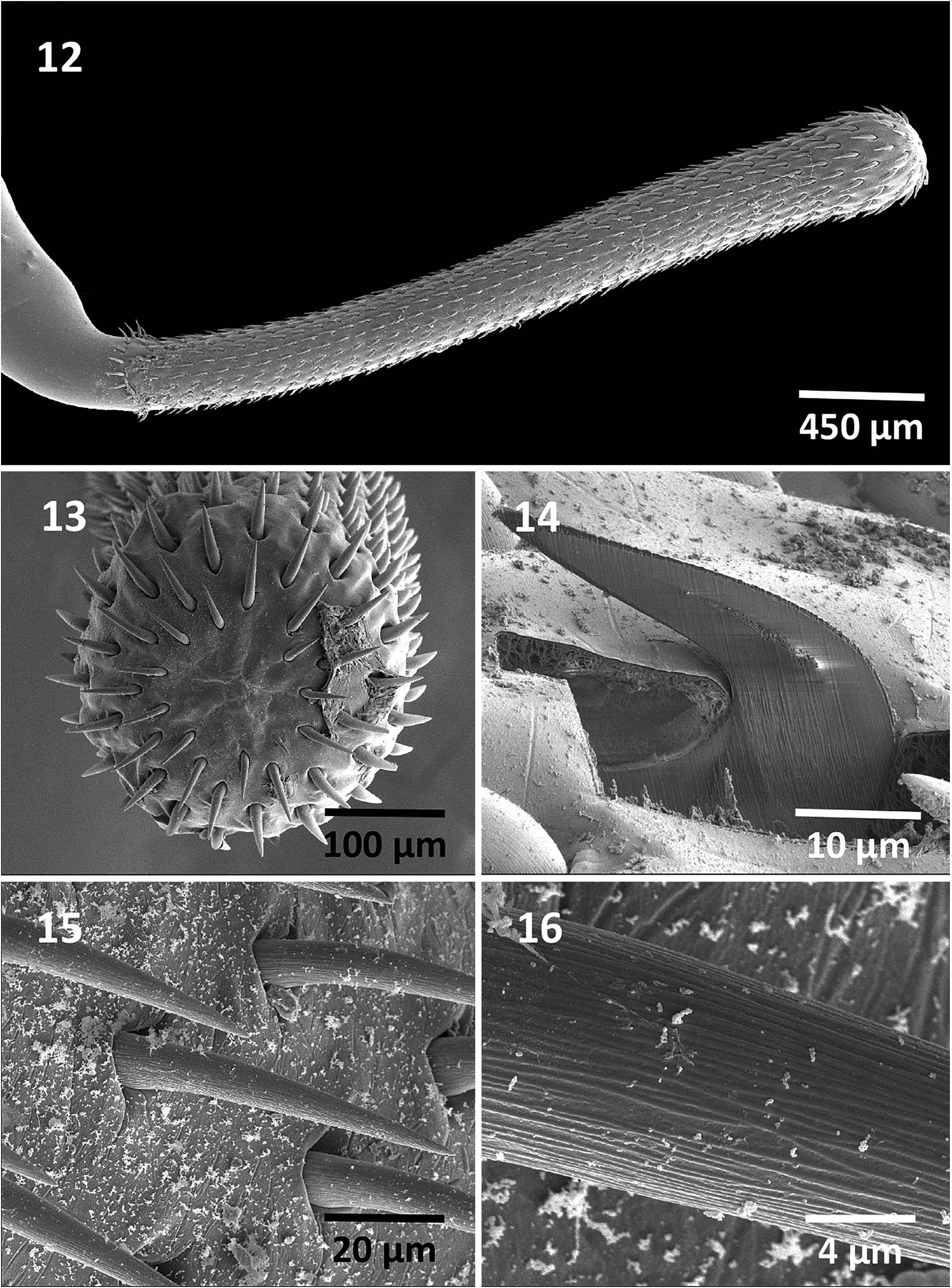
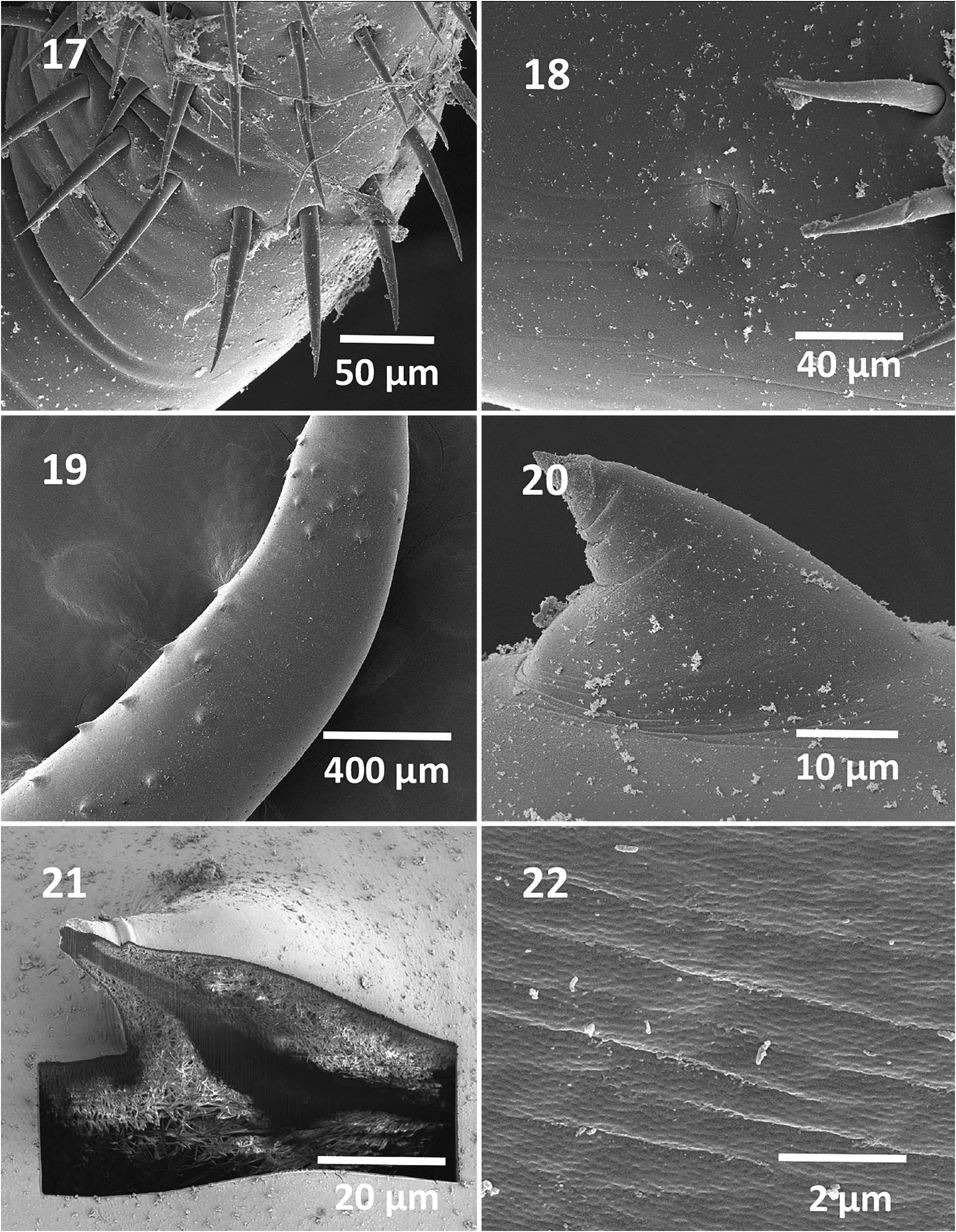
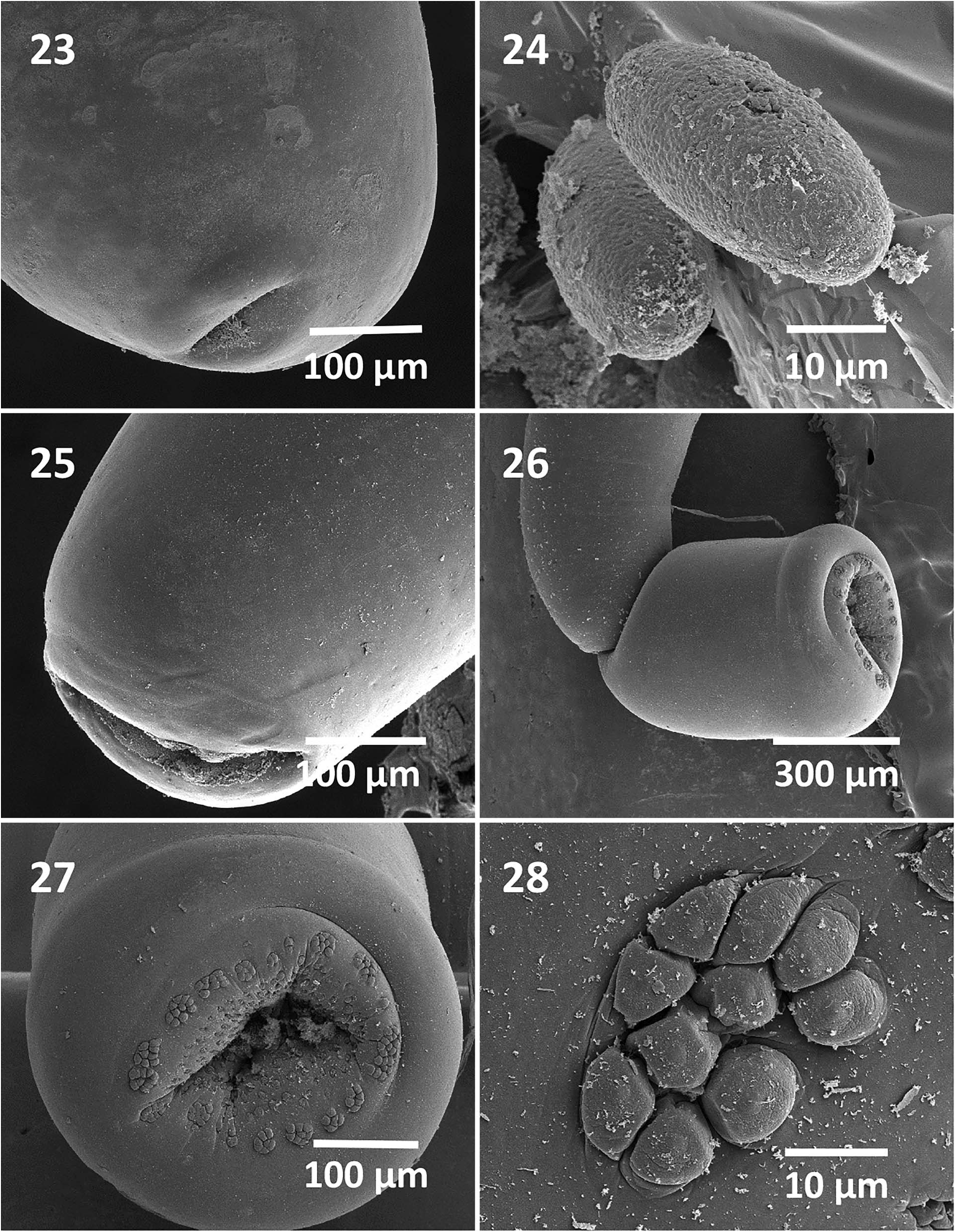
Rhadinorhynchus hiansi Soota and Bhattacharya, 1981 View in CoL View at ENA ( Figs. 1–31 View Figures 1–11 View Figures 12–16 View Figures 17–22 View Figures 23–28 View Figure 29 View Figure 30 View Figure 31 )
The present report represents a dramatic extension of the host and geographical distribution of a rhadinorhynchid acanthocephalan, R. hiansi , reported only once from 2 specimens collected from a flat needle fish, A. hians (Belonida) , off the southwestern coast of India almost 40 yr ago and provides a full morphological and molecular description of that species for the first time. Rhadinorhynchus hiansi was originally described from 2 male specimens collected from the flat needlefish Ablennes hians Valenciennes (Belonidae) off Trivandrum, Kerala, on the southwestern coast of India. Of the 129 specimens of R. hiansi from 9 of 10 striped bonito, S. orientalis off the southern Pacific coast of Vietnam at Nha Tran, we processed and whole mounted 32 specimens (19 males and 13 females) for microscopic examination, 15 specimens for SEM studies, 8 specimens for metal analysis of hooks, and 4 specimens for molecular studies. The remaining specimens are in ethanol in the Omar M. Amin collection .
General: With characters of the genus Rhadinorhynchus Lühe 1911 . Trunk relatively long, uniformly cylindrical, spinose anteriorly in 2 regions within range of proboscis receptacle ( Figs. 1, 2 View Figures 1–11 , 19 View Figures 17–22 ). Trunk and all shared structures markedly larger in females than in males with rare males almost reaching length of longest females. Cuticular surface flat with many electron-dense micropores ( Fig. 22 View Figures 17–22 ). Trunk spines with prominent dense central core ( Figs. 4 View Figures 1–11 , 20, 21 View Figures 17–22 ) (counted on 1 side of trunk) larger and usually more numerous in females than in males. Anterior trunk spines in near complete circles, fewer dorsally, not exceeding 5 vertically or horizontally. Posterior trunk spines reaching 9 ventrally and 11 laterally, larger at middle ( Table I View Table I ). Posterior lateral trunk spines smaller than posterior ventral spines and closer to ventral side of worms. Proboscis long, cylindrical, straight, titling ventrad ( Figs. 1, 2, 5 View Figures 1–11 , 12 View Figures 12–16 ), gradually widening and rounded anteriorly, with 21–24 longitudinal alternating rows ( Fig. 14 View Figures 12–16 ) of 36–48 hooks each ( Figs. 5 View Figures 1–11 , 12 View Figures 12–16 ), varying with worm sex. Hooks with thin cortical layer and thick solid core ( Fig. 19 View Figures 17–22 ), directed posteriorly with external striations (6, 7, 15, 16). Dorsal hooks slightly longer and slenderer than stouter ventral hooks especially in anterior half of proboscis ( Figs. 6, 7 View Figures 1–11 ). Apical hooks smallest ( Fig. 14 View Figures 12–16 ), third and forth hooks largest and thickest. Subsequent hooks progressively smaller posteriorly and then increasing in size nearing basal hooks. Basal crown hooks longest ( Fig. 17 View Figures 17–22 ) but slender and larger ventrally than dorsally (Table II; Figs. 6, 7 View Figures 1–11 ). Hook roots simple, relatively shorter than blades, directed posteriorly except few larger posterior 5 or 6 hooks having small roots with short anterior manubria (Table II; Figs. 6, 7 View Figures 1–11 ). Neck prominent, longer dorsally than wide posteriorly with paired sensory pores ( Fig. 18 View Figures 17–22 ). Proboscis receptacle about twice as long as proboscis, double-walled, about twice as long as proboscis with cephalic ganglion near its middle. Lemnisci digitiform, equal, uniformly broad throughout, usually slightly shorter than receptacle ( Figs. 1, 2 View Figures 1–11 ) but occasionally longer. Gonopore terminal in males and subterminal in females to various degrees ( Figs. 8, 10 View Figures 1–11 , 23 View Figures 23–28 ).
Males (based on 19 adults with sperm from S. orientalis ): Trunk 4.75–11.25 (7.04) mm long by 0.45–0.87 (0.58) mm wide at middle. See Table I View Table I for position, distribution, and size of trunk spines. Proboscis 1.62–2.20 (1.88) long by 0.19–0.28 (0.23) mm wide anteriorly. See Table II for measurements of proboscis hooks and roots. Neck 312–520 (418) long dorsally by 250–302 (279) wide posteriorly. Proboscis receptacle 3.50–4.87 (3.86) mm long by 0.22–0.32 (0.26) mm wide. Lemnisci 2.30–4.37 (3.24) mm long by 0.06–0.21 (0.13) mm wide. Reproductive system in posterior-most third of trunk in contiguous structures with genitalia opening into bursa ( Figs. 1, 3 View Figures 1–11 ). Testes ovoid; anterior testis 0.57–1.25 (0.77) mm long by 0.21–0.50 (0.33) mm wide, larger than posterior testis 0.42–1.12 (0.63) mm long by 0.21–0.55 (0.33) wide. Cement glands 4, rod-shaped, in 2 contiguous pairs, longer posteriorly ( Fig. 3 View Figures 1–11 ). Anterior glands 0.36–1.04 (0.87) mm long by 0.34–0.56 (0.21) wide; posterior glands 0.94–1.92 (1.50) mm long by 0.16–0.21 (0.18) mm wide. Individual cement gland ducts surround prominent fusiform Saefftigen’s pouch, 312–832 (489) mm long by 146–281 (193) mm wide, anteriorly and joining its posterior duct at thick-walled bursa ( Figs. 1, 3 View Figures 1–11 ). Bursa thickwalled with many sensory papillae in inner rings and in outer clusters ( Figs. 26–28 View Figures 23–28 ), 520 by 364 in 1 specimen.
Females (based on 13 mature females with eggs and ovarian balls from S. orientalis ): Trunk 10.50–24.75 (17.83) mm long by 0.42– 0.90 (0.61) mm wide at middle. See Table I View Table I for position, distribution and size of trunk spines. Proboscis 2.10–2.37 (2.30) mm long by 0.22–0.37 (0.27) mm wide anteriorly. See Table II for measurements of proboscis hooks and roots. Neck 416–520 (467) long dorsally by 250–322 (293) long by 109–300 (233) wide posteriorly. Neck 200–375 (315) long by 109–300 (233) wide posteriorly. Proboscis receptacle 3.00–5.75 (4.65) mm long by 0.20–0.32 (0.27) mm wide. Lemnisci 2.57–5.07 (3.74) mm long by 0.11–0.21 (0.13) wide. Reproductive system 2.60–6.12 (4.23) mm long (30% of trunk length: 25% in shortest worm and 29% in longest worm) ( Fig. 8 View Figures 1–11 ), with well-defined subterminal vagina ( Figs. 10 View Figures 1–11 , 23 View Figures 23–28 ), very long uterus, small and elongated uterine bell with few cells ( Figs. 8, 9 View Figures 1–11 ). Eggs elliptic with thick coarse outer shell, without polar prolongation of fertilization membrane ( Figs. 11 View Figures 1–11 , 24 View Figures 23–28 ), 37–62 (50) long by 15–23 (20) wide.
Taxonomic summary
Type host: Flat needlefish Ablennes hians Valenciennes (Belonidae) .
Other hosts: Striped bonito Sarda orientalis Temminck and Schlegel (Scombridae) ; this paper.
Type locality: Trivandrum , Kerala, India (08°29 ′ 15 ′′ N, 76°57 ′ 9 ′′ E) GoogleMaps .
Other localities: Southern Pacific coast of Vietnam at Nha Trang (12°15 ′ N, 109°11 ′ E). GoogleMaps Allotype female from Vietnam: HWML collection no. 139894 . GoogleMaps
Specimens: HWML collection no. 139895 (voucher males and females). GenBank accession nos: Rhhi18S1-S3: MN203133 View Materials - MN203135 View Materials ; RhhiCOI1-3: MN203136 View Materials - MN203138 View Materials . Z.S.I.Reg. No. WN 360/1 (holotype male and paratype male on same slide from Albennes hians at Trivandrum ) (Soota and Bhattacharya, 1981).
Remarks
The collection of a large number of specimens from S. orientalis along the Pacific coast of Vietnam provided an opportunity to fully describe males and females of R. hiansi for the first time. The description of 2 males by Soota and Bhattacharya (1981) did not include complete text and/or illustrations information on the proboscis receptacle, trunk spines, neck, lemnisci, or cement glands and made no reference to hook roots or Saefftigen’s pouch. The range of measurements was limited by the number of specimens that they examined (2). Irrespective, it appears that their specimens had smaller trunks (8.8–10.00 mm long) and somewhat longer proboscides (2.2–2.25 mm long). Their specimens had shorter lemnisci (2.5–2.6 mm long), anterior testis (470–490 long), and posterior testis (440– 460 long). Soota and Bhattacharya (1981) also indicated the length of the thinner dorsal and the thicker ventral hooks as 33– 66 and 33–55 long, respectively. Our specimens were, however, readily recognizable as R. hiansi and are fully described and illustrated.
Energy Dispersive X-ray Analysis
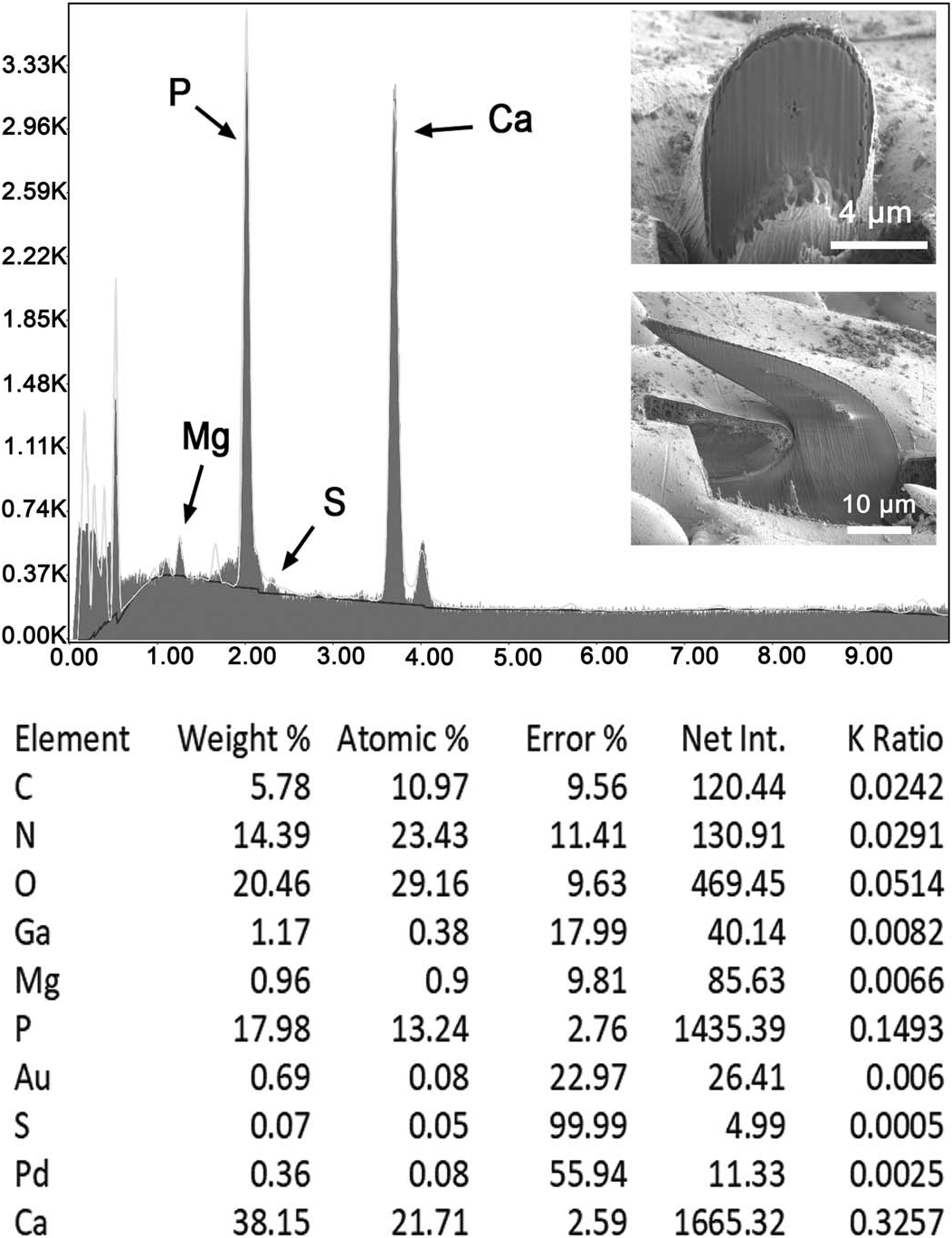
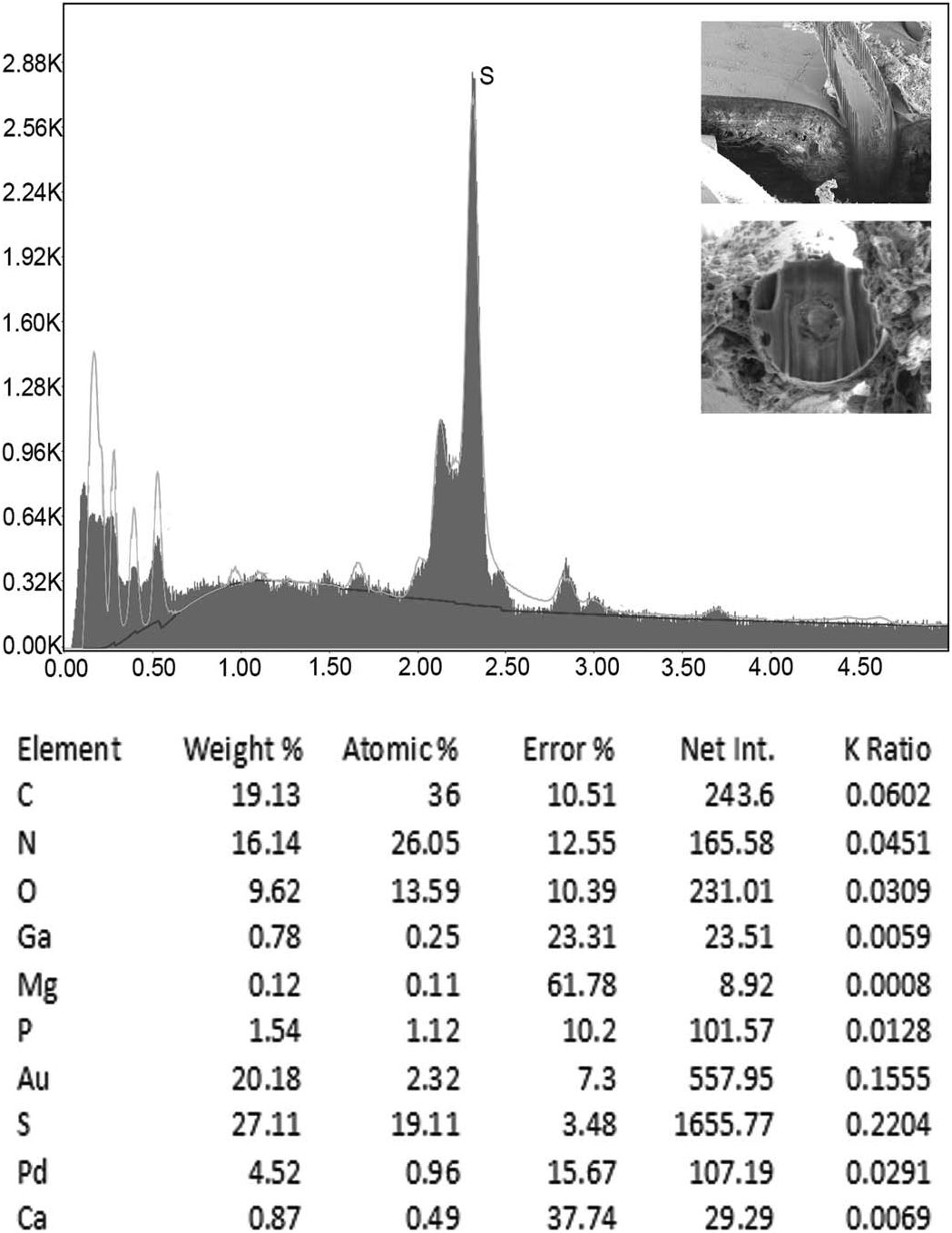
The results of large and small gallium (Ga) cut hooks and trunk spines are given in Tables III–V and represented by Figures 29– 31 View Figure 29 View Figure 30 View Figure 31 . The elements necessary for the mineralization and hardening of the hooks and spines especially calcium and phosphorus are present with sulfur, especially highest in small hooks and trunk spines.
Molecular analyses
Three partial 18S rDNA (742–764 nt) and 3 COI (619–628 nt) sequences were generated from 4 adult specimens of R. hiansi . Two 18S rDNA sequences were identical (and thus only one of them was thus included in the corresponding phylogenetic tree), and, with respect to the third one, intraspecific divergence was 0.003% (2 nt difference). Intraspecific sequence divergence for the COI gene ranged between 0.002 –0.024 % (1–12 nt difference).
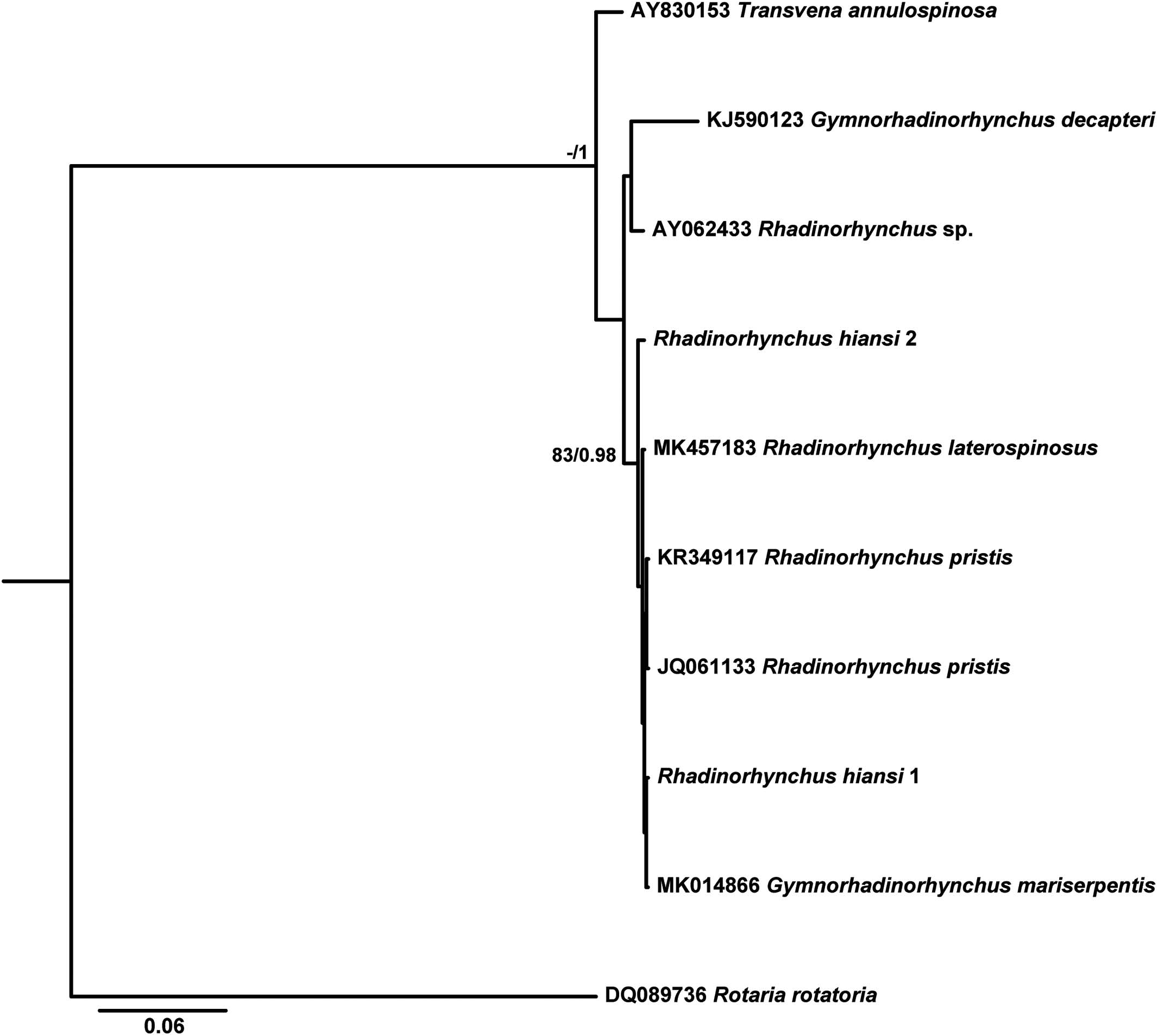
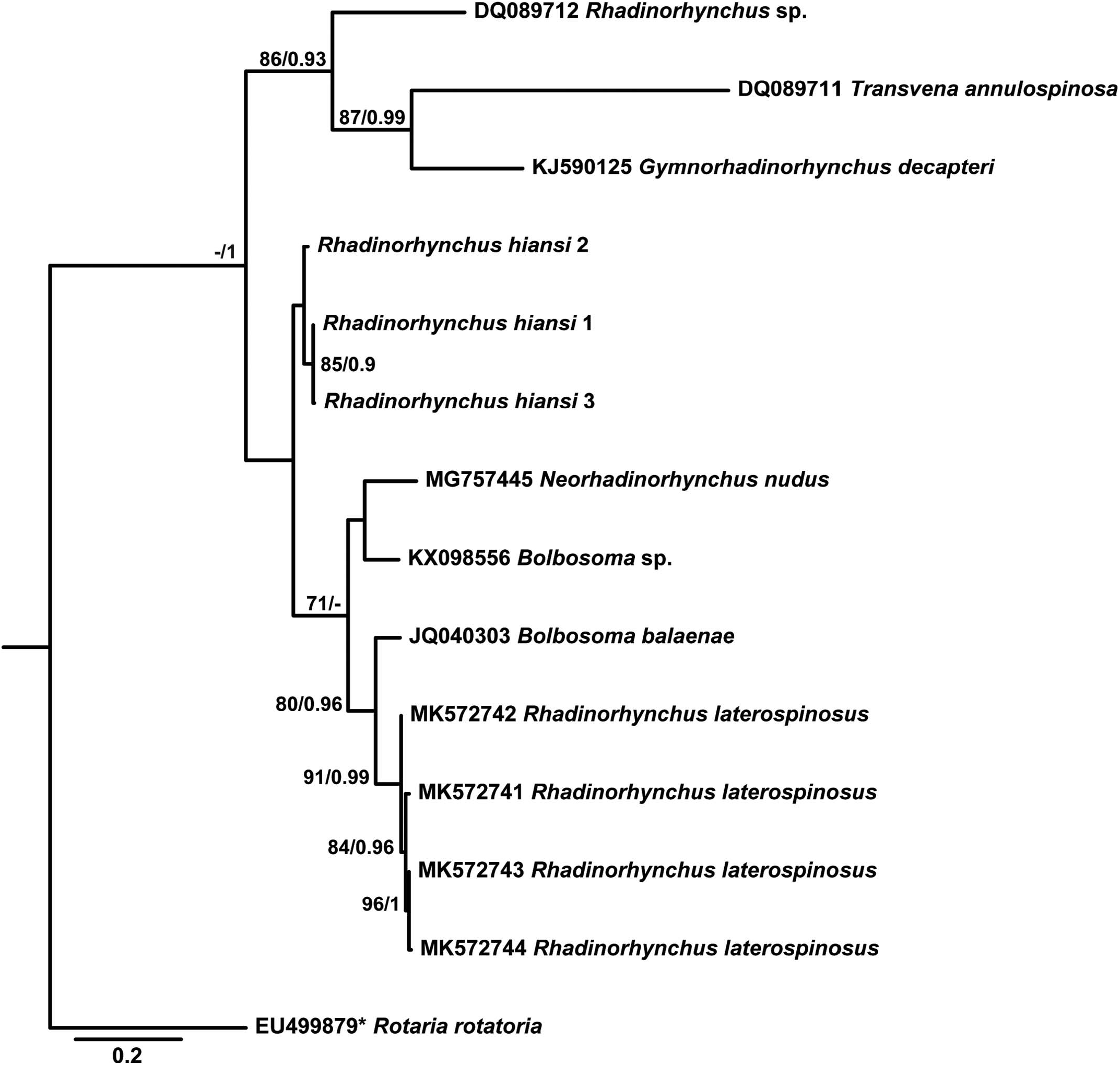
Table VI provides data for the sequences retrieved from GenBank and used in the phylogenetic analyses based on the 2 alignments. Since ML and BI algorithms produced trees with identical topology for the 2 sequenced genes, only the BI tree is shown for 18S ( Fig. 32 View Figure 32 ) and the ML tree for the COI gene ( Fig. 33 View Figure 33 ).
In the phylogenetic tree based on 18S rDNA, R. hiansi formed a highly supported clade with the congeneric species Rhadinorhynchus pristis (Rudolphi, 1802) and Rhadinorhynchus laterospinosus Amin, Heckmann and Ha, 2011 , and also with Gymnorhadinorhynchus mariserpentis Steinauer, Garcia-Vedrenne, Weinstein and Kuris, 2019 (0.000 –0.003 %, 0–2 nt difference from newly generated sequences in all cases). Less related to R. hiansi was a sequence belonging to an unknown species of Rhadinorhynchus (0.013%, 10 nt difference) that grouped, with low support, with Gymnorhadinorhynchus decapteri Braicovich, Lanfranchi, Farber, Marvaldi, Luque and Timi, 2014 (0.039 and 0.040%, 29 and 30 nt difference from newly generated sequences).
According to phylogenetic analyses based on the COI gene, the 3 newly generated sequences for R. hiansi were sisters to a group including sequences of R. laterospinosus (0.130 –0.136 %, 66–69 nt difference) as well as representatives of Bolbosoma and the species Neorhadinorhynchus nudus (Harada, 1938) Yamaguti, 1939 (0.130 –0.149 %, 66–76 nt difference from newly generated sequences). Separately, it remained a clade including a sequence assigned to the same unknown species of Rhadinorhynchus present in the 18S phylogeny (0.234%, 119 nt difference from newly generated sequences) and which grouped with G. decapteri and Transvena annulospinosa Pichelin and Cribb, 2001 (0.250 –0.312 %, 127–159 nt difference from newly generated sequences).
Table I. Size and distribution of trunk spines of 19 male and 13 female specimens of Rhadinorhychus hiansi from Sarda orientalis in Vietnam.
| Anterior trunk spines | Posterior trunk spines | |||
|---|---|---|---|---|
| Dorsal | Ventral | Across | Ventral | Lateral |
| Males | ||||
| 0–3 (1.1)* 31–62 (47.3)* | 0–4 (2.5) 31–62 (47.3) | 2–5 (3.0) 31–62 (47.3) | 2–7 (5.0) 42–73 (61.5) | 0–12 (5.0) 35–52 (43.8) |
| Females | ||||
| 0–4 (1.6) 52–73 (62.3) | 1–4 (2.6) 52–73 (62.3) | 0–5 (3.5) 52–73 (62.3) | 5–9 (6.7) 62–84 (71.1) | 0–11 (4.3) 52n73 (64.4) |
* Range of number of spines on 1 side of worms (mean); range and mean of spine length in micrometers.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |