Etmopterus lii, Ng & Liu & Joung, 2024
|
publication ID |
https://doi.org/ 10.26107/RBZ-2024-0002 |
|
publication LSID |
lsid:zoobank.org:pub:DF32BB8A-0012-4583-BAB8-4A79C039F157 |
|
persistent identifier |
https://treatment.plazi.org/id/59792205-5AC5-4EA8-81F4-34195BD7F9CE |
|
taxon LSID |
lsid:zoobank.org:act:59792205-5AC5-4EA8-81F4-34195BD7F9CE |
|
treatment provided by |
Felipe |
|
scientific name |
Etmopterus lii |
| status |
sp. nov. |
Etmopterus lii , new species
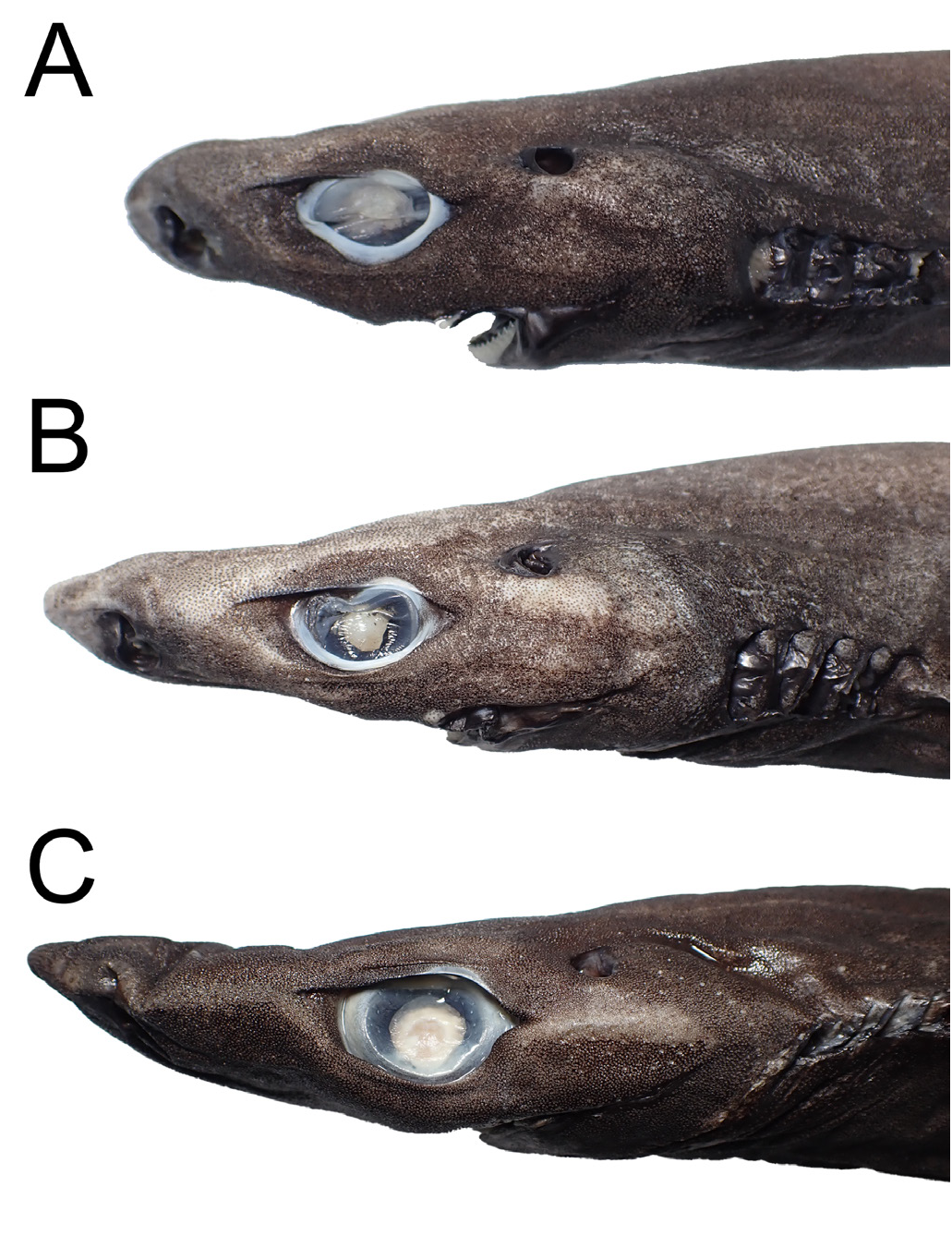
Li’s lanternshark ( Figs. 2 View Fig , 3A, B View Fig , 4 View Fig , 5A View Fig , 6A, C View Fig , 7–8 View Fig View Fig ; Tables 1–3)
Holotype. ASIZP0081745 View Materials , 341 mm TL, mature male, South China Sea (ca. 19° N, 114° E), ca. 500 m depth, 12 March 2023. GoogleMaps
Paratypes. 22 specimens: ASIZP0081739 View Materials , 129 mm TL, immature male, ASIZP0081740 View Materials , 252 mm TL, immature male, South China Sea (ca. 19° N, 114° E), ca. 500 m depth, 25 March 2022, C.-H. Lin; ASIZP0081741 View Materials , 143 mm TL, immature female, South China Sea (ca. 19° N, 114° E), ca. 500 m depth, 31 May 2022; ASIZP0081742 View Materials , 381 mm TL, immature female, ASIZP0081743 View Materials , 301 mm TL, immature male, ASIZP0081744 View Materials , 325 mm TL, mature male, collected with the holotype; NMMB-P039251 , 127 mm TL, immature male, NMMB-P039252 , 119 mm TL, immature female, NMMB-P039253 , 228 mm TL, immature female, South China Sea (ca. 19° N, 114° E), ca. 500 m depth, 25 March 2022; NMMB-P039254 , 176 mm TL, immature female, NMMB-P039255 , 231 mm TL, immature male, South China Sea (ca. 19° N, 114° E), ca. 500 m depth, 25 April 2022; NMMB-P039256 , 195 mm TL, immature male, NMMB-P039257 , 126 mm TL, immature male, South China Sea (ca. 19° N, 114° E), ca. 500 m depth, 13 May 2022; NMMB-P039258 , 353 mm TL, immature female, NMMB-P039259 , 361 mm TL, immature female, NMMB-P039260 , 335 mm TL, immature female, NMMB-P039261 , 321 mm TL,mature male, NMMB-P039262 , 288 mm TL, immature male, NMMB-P039263 , 271 mm TL, immature male, NMMB-P039264 , 315 mm TL, immature male, NMMB-P039265 , 325 mm TL, mature male, NMMB-P039266 , 332 mm TL, mature male, collected with the holotype GoogleMaps .
Diagnosis. A medium-sized species of Etmopterus differing from all other congeners except E. sheikoi , by having a combination of flat and frustum-shaped denticles, elongated anterior and posterior lateral flank markings, and multicuspid lower jaw teeth in mature males. It differs from E. sheikoi by having a much narrower posterior flank marking, the shape and position of the caudal base marking, the length of posterior caudal marking, relatively larger gill slits, relatively more monospondylous, precaudal and total centra.
Description. Measurements are listed in Table 2. Values are expressed as a percentage of total length (TL) for the holotype, followed by the range of values for 22 paratypes in parentheses.
Body fusiform ( Fig. 2 View Fig ), trunk sub-cylindrical, width 78.5 (36.9–88.3) % height; abdomen longer than lower caudal peduncle, pectoral-pelvic space 144.5 (102.5–193.5) % pelvic-caudal space; head subconical, length 24.3 (23.1–27.1) % TL, slightly depressed, height 80.6 (61.0–115.8) % width. Snout moderately long (very long in some paratypes), preorbital length 7.0 (6.1–8.7) % TL, 28.8 (25.0–33.8) % head length, 160.6 (128.5–215.9) % orbit length; snout bluntly rounded to slightly pointed in lateral view ( Fig. 3A, B View Fig ), narrowly rounded in dorsal view. Eyes oval, orbit width 57.8 (46.4–78.6) % height; orbits with both anterior and posterior notches; eyes narrowly spaced, interorbital width 73.8 (59.3–85.8) % head width, orbit length 60.2 (51.0–80.5) % interorbital width. Spiracles small, bean-shaped, length 34.2 (21.8–45.3) % orbit length, 6.1 (3.7–9.8) % head length. Nostrils oblique, length 71.3 (59.3–135.5) % internarial width, 45.2 (35.7–71.6) % orbit length; anterior nasal flap narrowly triangular, tip just reaching the nasal opening, length 40.7 (22.2–61.7) % nostril width. Gill openings large, nearly straight, intergill length 4.7 (2.4–7.8) % TL, gill-slits height 1.6–1.8 (1.3–3.0) % TL. Mouth broad, length 99.0 (73.0–173.4) % width, very slightly arched.
free rear tips, base narrow, 55.2 (42.4–66.8) % pectoral-fin length, posterior margin slightly concave. Pelvic fin narrowly triangular, height 33.0 (21.2–47.8) % length. Clasper of mature males rather long, inner length 77.2 (68.1–78.7) % pelvic-fin length. Caudal fin elongate, dorsal length 23.5 (19.5–27.0) % TL; caudal folk very poorly developed, lower postventral margin 34.3 (15.1–54.2) % upper postventral margin; terminal lobe broad.
Dermal denticles frustum-shaped, small, flat, very closely spaced, giving a smooth texture of the skin, not in defined rows ( Fig. 5 View Fig ); denticles present on underside of snout, except for a narrow area around mouth; underside of gill slits with a V-shaped naked area, connecting gill slits between both lateral sides; inner margin of fins with very narrow naked area (inner margin of pectoral fin with a moderately large naked area in immature individuals); denticles present on fin bases, but absent on ceratotrichia.
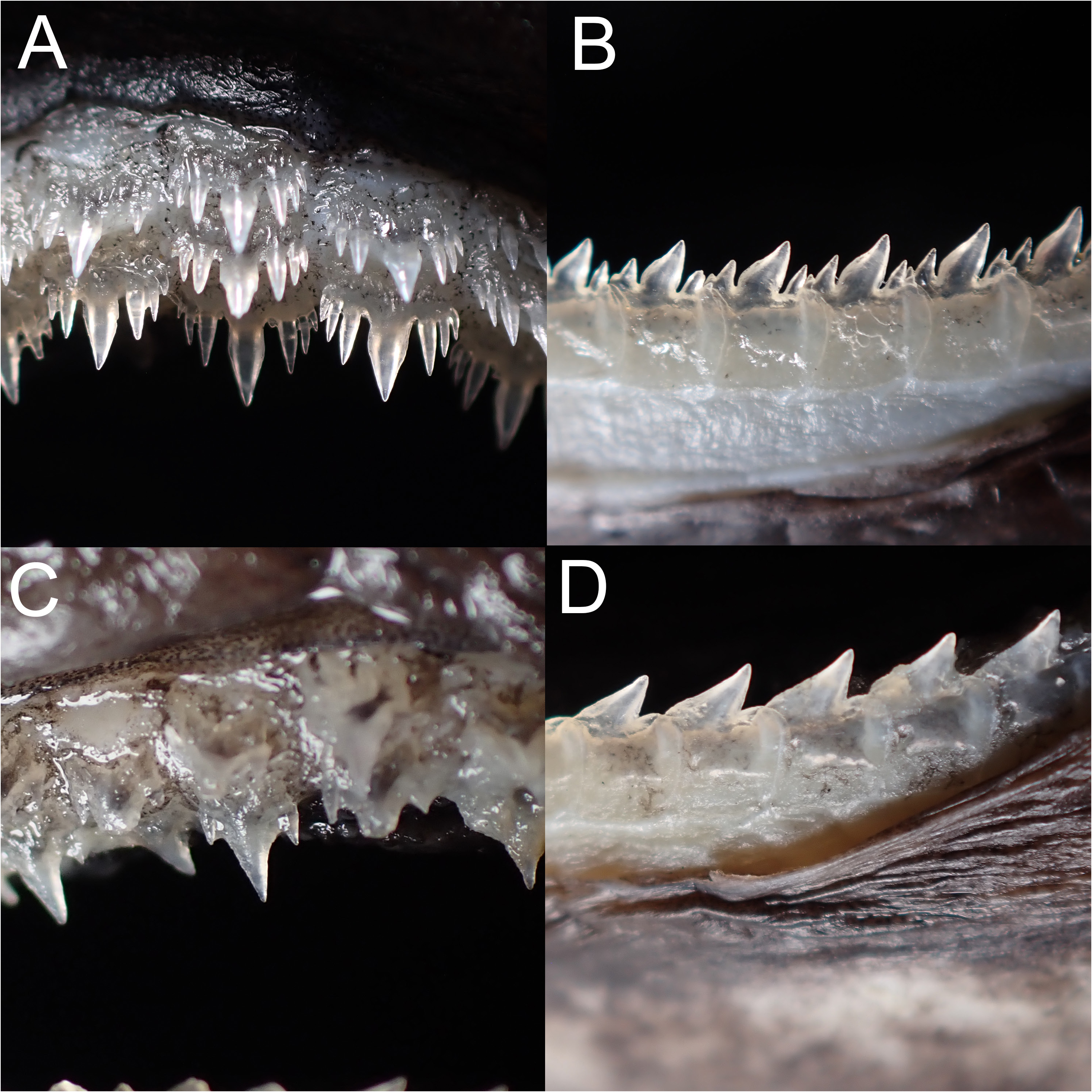
Teeth dissimilar in upper and lower jaw, with strong ontogenetic and sexual dimorphism ( Fig. 4 View Fig ); upper teeth multicuspid, in three functional series, small, central cusp thick; immature males and females with two cusplets on each side of the cusp of upper teeth, while mature males have three cusplets; inner pair of cusplet longest, length about two-third of the central cusp; teeth in lower jaw unicuspid in immature individuals, in three series, one functional; lower teeth blade-like, with strongly oblique cusp; the cusps of lower teeth of mature males are flanked with two to three small cusplets on each side, the outer one minute. Tooth count of upper jaw 23 (21–25), lower jaw 30 (27–32), total count 53 (49–55).
First dorsal fin long and large, with a round apex, length of first dorsal fin 12.0 (9.6–12.3) % TL, origin posterior to pectoral-fin free rear tip; pre–first dorsal fin length 174.9 (181.3–235.5) % interdorsal space; first dorsal–fin spine (140.1–250.6) % first dorsal-fin height. Second dorsal fin larger than first dorsal fin, first dorsal-fin height 81.1 (51.9–91.4) % second dorsal-fin height; apex angular, posterior margin especially concave, free rear tip moderately elongated; second dorsal-fin length 12.3 (11.2–15.2) % TL, interdorsal space 196.9 (124.8–219.4) % dorsal-caudal space; second dorsal–fin spine long and curved; second dorsal-fin origin just posterior to insertion of pelvic fins. Interdorsal space 85.9 (61.9–86.7) % pre–pectoral length. Pectoral fins moderate, length 10.1 (8.1–12.3) % TL, with angular Luminescent markings on head not distinct after frozen; head dorsal surface with a single line of dot-like markings, extending mid-dorsally from about the level of anterior fontanelle to the second dorsal-fin origin; ventral surface of pectoral fin with an arched marking, the tip not reaching the origin of pectoral-fin ceratotrichia; dash-like markings absent on lateral side. Pelvic-fin flank markings well defined in some specimens (sometimes difficult to inspect) ( Fig. 6A, C View Fig ), with elongated anterior and posterior branch; anterior branch rather short, length 9.2 (7.3–10.3) % TL, slender and straight, extending above pelvic–fin origin; posterior branch straight, thicker and shorter than anterior branch, length 60.8 (38.0–82.9) % length of anterior branch, width 0.9 (0.5–1.1) % TL, not extending beyond second dorsal-fin free rear tip; base of flank marking wide, base length 4.3 (2.9–5.2) % TL, origin slightly posterior to second dorsal-fin origin. Infracaudal marking barely visible, extending from the pelvic-fin flank marking base to about the same level of the posterior marking tip, not connecting to the caudal-fin base marking. Caudal-fin base marking thin, rod-like, oblique, moderately long, originate just before the lower caudal-fin origin, bifurcate after the origin, leaving a small black portion on the lower caudal-fin origin when viewed laterally. No central caudal-fin marking. Posterior caudal-fin marking short, its length 3.4 (2.3–5.7) % TL.
Vertebral counts: monospondylous 41 (38–43), diplospondylous precaudal 14 (12–17), caudal 26 (22–28), precaudal 55 (50–59), total 81 (72–84).
Colouration. After frozen, blackish grey to pale black, becoming dark black ventrally; transition between lateral and ventral sides not strongly demarcated. Dorsal midline without pale stripe; dash-like black markings absent on lateral side. Pectoral, pelvic and first dorsal fins generally dark grey to black, fin edges translucent, second dorsal fin black at fin base, becoming pale grey on ceratotrichia. Caudal fin dark grey, with black postventral margin. No dark blotch on caudal fin. No discernible blotch between infracaudal and caudal-fin base marking.
After preservation, body colouration becomes slightly paler; all the markings sometimes become less distinct ( Fig. 7 View Fig ).
Size. Up to 381 mm TL and 341 mm TL for females and males, respectively. Specimens smaller than 143 mm TL have umbilical scars, representing the approximate birth size.
Distribution. Known so far only from the northern South China Sea, at a depth of approximately 500 m.
Biological notes. All the females are immature, while the smallest mature male examined is 325 mm TL. The largest female we examined (ASIZP0081742) has developing ovaries and uterus, representing a maturing stage, suggesting that mature females apparently attain sizes larger than 400 mm TL. Some specimens have whole lanternfishes (family Myctophidae ) inside their stomachs, as observed under X-radiographs.
Etymology. The species is named after Mr. Yong-Tai Li, the captain of the fishing vessel Xin Yong Tai, for his assistance in not only obtaining the specimens in this study, but also many other deep-sea organisms from the South China Sea for other researchers, and thus making a great contribution to marine science research. Vernacular: Li’s lanternshark.
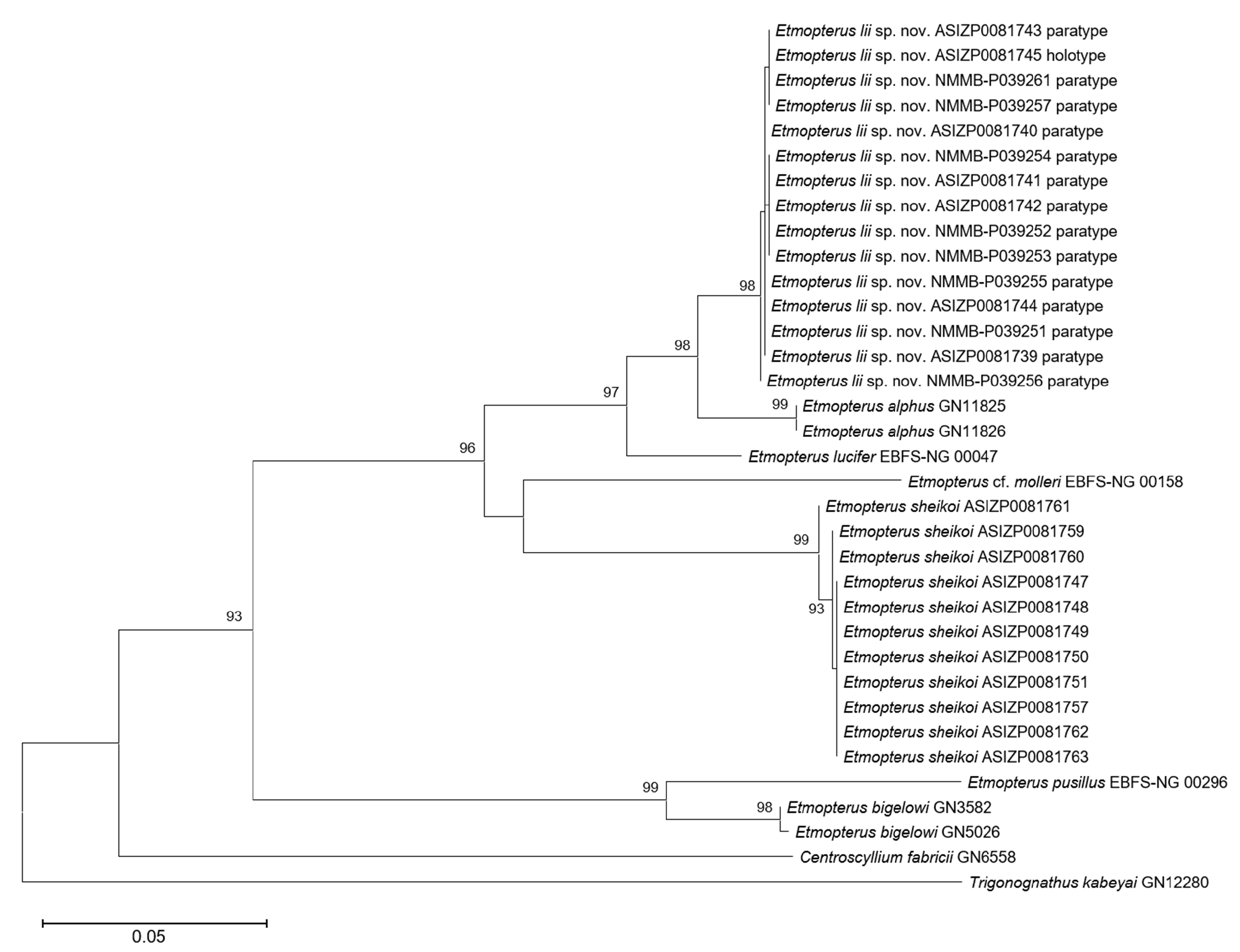
Remarks. By having both anterior and posterior branches elongated and based on the phylogenetic tree based on the structure of NADH2 sequences, Etmopterus lii is assigned to the E. lucifer group ( Straube et al., 2010). White et al. (2017) and Ebert et al. (2021) also considered the linear arrangement of dermal denticles a character of this group. However, this character is not only absent in E. lii , but also found in species of other groups (e.g. E. granulosus Compagno, 1984 ; E. splendidus Yano, 1988 ). Therefore, it should not be recognised as a shared character in the E. lucifer group.
The multicuspid lower teeth of the mature males of Etmopterus lii resembles the etmopterid genus Centroscyllium . However, all the immature males and females possess unicuspid lower teeth, which is not observed in Centroscyllium . Moreover, the molecular analysis shows that the new species clusters with other Etmopterus species instead of the Centroscyllium species, thus, our generic placement is supported by both morphology and genetics.
Ontogenetic and sexual dimorphism in tooth morphology has been documented in other lanternshark species (for example, E. granulosus, Straube et al., 2008 ; E. spinax, Straube & Pollerspöck, 2020 ; E. sheikoi, Adnet et al., 2006 , present study). Mature males usually display more cusplets on upper-jaw teeth and have a more erect cusp on lower-jaw teeth compared to other ontogenetic stages and females. The mature males of the new species also have more cusplets on each side of the cusp (3) than females and immature individuals (1–2). Notably, mature males of E. lii have multicuspid lower teeth, which is the second known Etmopterus species showing this character. However, the dimorphism in lower teeth of mature females is uncertain, as all the female specimens obtained so far are immature. The absence of mature females in our sampling may be due to sexual or habitat segregation with the males.
Comparisons. Within the E. lucifer group, Etmopterus lii is especially unique by having frustum-shaped dermal denticles and having muticuspid lower-jaw teeth in mature males, thus is not likely to be misidentified or confused with other members. The new species is most similar to E. sheikoi , which is also assigned to the E. lucifer group based on our phylogenetic reconstruction ( Fig. 1 View Fig ) (see remarks on E. sheikoi below), yet it can be readily distinguished from the latter by the following characters: a shorter snout, length 25.0–33.8% TL (vs. 34.5–40.9 % head length in E. sheikoi ), the origin of second dorsal fin anterior to flank-marking base origin (vs. the origin of second dorsal fin well posterior to flank-marking base origin), a narrower posterior branch of flank marking, width 0.5–1.1% TL (vs. 1.5–2.3% TL), a thin, oblique, rod-like caudal-fin base marking originating just before the lower caudal-fin origin (vs. a thick, flat caudal-fin base marking, originating well before the lower caudal-fin origin), and shorter posterior caudal-fin marking, length 2.3–5.7% TL (vs. 7.8–11.3% TL). The new species also possesses relatively larger gill slits ( Fig. 8 View Fig ) and has more monospondylous centra (38–43 vs. 42–45 in E. sheikoi ), precaudal centra (52–59 vs. 58–64), and total centra (76–84 vs. 85–93), although the above characters share a little overlap ( Table 3). In addition, E. lii has a smaller tooth count (21–25/27–32) than E. sheikoi specimens larger than 323 mm TL (32–48/34–44). That said, no ontogenetic changes in tooth count were observed in the former. When comparing the lower teeth of mature males, the inner pair of cusplets of E. lii is much shorter, with the length about half of the cusp, while in E. sheikoi the inner pair of cusplets (2 nd pair) is about two-thirds to about the same height of the cusp.
Etmopterus lii may also be confused with the only two other congeners possessing frustum-shaped dermal denticles, E. bigelowi and E. pusillus , yet it differs from the two species by having elongated posterior flank-marking branches (absent in both species), a much shorter caudal-fin base marking, length 5.3–8.9% TL (vs. 13.6–18.0% TL in E. bigelowi ; 11.6% TL in E. pusillus ), an arched pectoral-fin marking (vs. subrhombic-shaped in both species), much fewer monospondylous centra (38–43 vs. 53–55 in E. bigelowi , 52 in E. pusillus ), and more diplospondylous trunk centra (12–17 vs. 7–12 in E. bigelowi , 9 in E. pusillus ).
Although sharing a similar genetic structure with E. alphus , E. lii is easily distinguished from the former by having frustum-shaped denticles (vs. hook-like denticles in E. alphus ), shorter posterior caudal-fin marking (2.3–5.4 vs. 7.0–8.2% TL), pelvic-fin flank marking base origin posterior to second-dorsal fin origin (vs. just anterior to second dorsal-fin origin), and lateral and ventral side of body not demarcated (vs. strongly demarcated in both fresh and preserved condition).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |