Dinoponera grandis ( Guérin-Méneville, 1838 )
|
publication ID |
https://doi.org/10.5852/ejt.2021.784.1603 |
|
publication LSID |
lsid:zoobank.org:pub:80B6E154-A9A3-49E3-AAF0-3FD2BEBF82D2 |
|
DOI |
https://doi.org/10.5281/zenodo.5799535 |
|
persistent identifier |
https://treatment.plazi.org/id/096C6310-A100-FFEE-FE64-A251FDD1F148 |
|
treatment provided by |
Felipe (2021-12-22 14:00:53, last updated 2024-12-04 17:16:48) |
|
scientific name |
Dinoponera grandis ( Guérin-Méneville, 1838 ) |
| status |
|
Dinoponera grandis ( Guérin-Méneville, 1838) . Revived species
Figs 15–18 View Fig View Fig View Fig View Fig , 28A View Fig
Ponera grandis Guérin-Méneville, 1838: 206 (☿).
Dinoponera grandis australis Emery, 1901: 48 View in CoL (☿). syn. nov.
Dinoponera australis brevis Borgmeier, 1937: 227 (☿).
Dinoponera australis bucki Borgmeier, 1937: 228 View in CoL , fig. 7 (☿, ♂). syn. nov.
Dinoponera australis nigricolor Borgmeier, 1937: 228 View in CoL , figs 5–6, 8 (☿, ♂). syn. nov.
Dinoponera snellingi Lenhart, Dash & Mackay, 2013: 152 View Cited Treatment , figs 4d, i, n, 5b, 7, 9b, 10b, 11b (♂). syn. nov.
Dinoponera grandis – Roger 1861: 38 (combined in Dinoponera View in CoL , synonym of D. gigantea View in CoL ). — Bequaert 1926: 188 (junior synonym of D. gigantea View in CoL ).
Dinoponera grandis australis View in CoL – Santschi 1921: 85 (male description). — Borgmeier 1937: 227 (raised to species, lectotype designated). — Kempf 1975: 382, figs 6, 17–18 (redescription, key). — Lenhart, Dash & Mackay 2013: 135, figs 4e, j, o, 5d, 9d, 10d, 11d (redescription, male genitalia, key). — Tozetto & Lattke 2020: 5, figs 2–3, 6 (male genitals).
Dinoponera australis brevis – Kempf 1971: 382 (junior synonym of D. australis View in CoL ).
Dinoponera australis nigricolor View in CoL – Kempf 1971: 387 (lectotype male designated).
Non Dinoponera grandis australis View in CoL brevis – Santschi 1928: 416 (invalid name).
Diagnosis
Female
Malar area with longitudinal to oblique striae that reach anterior ocular margin. Abdominal tergite III microareolate and opaque to slightly silky, never smooth and shining. Hind basitarsus length less than 6 mm long. Petiolar node short, DPI ≥ 0.8. BL≤ 27 mm.
Male
Body, including antenna, without long hairs. Basal ring in lateral view with dorsal margin declining and extending anteriorly to form a broad lobe. Penisvalva in lateral view with a posterior rounded apex, projecting ventrolaterally.
Material examined
Lectotype of Ponera grandis BRAZIL – Minas Gerais • ☿; ZSM.
Other type specimens (13 ☿☿, 2 ♂♂)
BRAZIL – Goiás • ♂, lectotype of D. australis nigricolor ; Campinas; 5 May 1933; Schwarzmaier leg.; MZSP 65801 • 1 ☿, paralectotype of D. australis nigricolor ; Campinas ; 6–8 Feb. 1936; R. Spitz leg.; MZSP 67063 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67064 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67066 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67067 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67068 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67069 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67070 • 1 ☿, paralectotype of D. australis nigricolor ; same locality as for preceding; 4 Nov. 1937; Schwarzmaier leg.; MZSP 67071 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67072 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67073 • 1 ☿, paralectotype of D. australis nigricolor ; same collection data as for preceding; MZSP 67074 . – Rio Grande do Sul • ♂, lectotype of Dinoponera australis bucki (here designated); Palmeira das Missões; 27 Jan. 1929; P. Buck leg.; MZSP 65800 • 1 ☿, paralectotype of D. australis bucki ; same collection data as for preceding; MZSP 67061 • 1☿, paralectotype of D. australis bucki ; same collection data as for preceding; MZSP 67062 .
Non-type specimens (234 ☿☿, 9 ♂♂)
ARGENTINA – Missiones • 1 ☿; Bruch leg.; MZSP • 2 ☿☿; Iguazú; Oct. 1964; A. Martinez leg.; MZSP .
BOLÍVIA – Icnilo • 1 ☿; Buenavista ; Feb. 1950; A. Martinez leg.; MZSP . – Santa Cruz • 1 ☿; Gutierrez, Nueva Moka [ Moca ]; 23 Nov. 1951; A. Martinez leg.; MZSP • 1 ☿; “ Samaipata - balneário mama Pascula ”; 3 Jan. 1989; F.J.A. Peralta and Hansen leg.; INPA • 1 ☿; San José de Chiquitos ; 3–5 Mar. 1954; C. Gans and F. Pereira leg.; MZSP • 1 ☿; Sara, Nueva Moka ; Feb. 1950; A. Martinez leg.; MZSP • 1 ☿; Nueva Moka; Mar. 1956; A. Martinez leg.; MZSP.
BRAZIL – Distrito Federal • 1 ☿; APA Gama Cabeça de Veado ; 2 Mar. 2000; Pic. Mireille leg.; CPDC • 2 ☿☿; Brasília ; Oct. 1961; R.L. Araújo leg.; MZSP • 1 ☿; Brasília ; 15.96565° S, 47.91626° W; 8 Apr. 2011; H.L. Vasconcelos and T. Frizzo leg.; 1157, T5-6S; UFU GoogleMaps • 1 ☿; Brasília, Ceilândia ; 15 Oct. 1976; 499; DZUP • 1 ☿; Brasília , E. Ecol. Águas Emendadas; 15 Dec. 1992; A. Reis leg.; MZSP • 1 ☿; same locality as for preceding; 19 May 1993; J.H. Schoereder leg.; UFV LabEcol 437 • 1 ☿; same collection data as for preceding; UFV LabEcol 438 • 1 ☿; Brasília , FAL ; 15°57′47.4″ S, 47°55′35.7″ W; 16 Oct. 2010; M.C. Galego-Ropero and P. Rezende leg.; “ cupinzeiro C. cumulans ”; MZSP GoogleMaps • 1☿; Brasília, Reserva Ecológica do IBGE (RECOR) , Fisionomia campo limpo ; 15.9166° S, 47.8666° W to 15.9500° S, 47.8833° W; 2009; F.A. Schmidt leg.; P:5 E:E; UFV LabEcol 445 GoogleMaps • 1 ☿; Brasília, Reserva Ecológica do IBGE , cerrado sensu stricto ; 6 Feb. 2008; J. Maravalhas leg.; MZSP • 1 ☿; same collection data as for preceding; 7 Feb. 2008; MZSP • 1 ☿; UnB Campus ; 10 Nov. 1974; Bittencourt leg.; 11756, 500; DZUP • 1 ☿; same collection data as for preceding; 11756, 501; DZUP . – Goiás • 1 ☿; 7 km NW of Alto Paraíso, Morro das Cobras ; 1–7 Jul. 1991; C.R.F. Brandão, M.L. Françoso and A.A. Reis leg.; pitfall stn 1; MZSP • 1 ☿; Abadia de Goiás ; 6 Nov. 1980; C.R. Krigger leg.; UFSC • 1 ☿; Anápolis ; Nov. 1938; M. Souza leg.; MZSP • 1 ☿; Anápolis, UEG , Campus CCET ; 16.3826° S, 48.9446° W; 5 Oct. 2010; H.F. Cunha leg.; UEG GoogleMaps • 6 ☿☿; same collection data as for preceding; 18 Oct. 2010; UEG GoogleMaps • 1 ☿; same collection data as for preceding; 20 Oct. 2010; UEG GoogleMaps • 1☿; same collection data as for preceding; 27 Oct. 2010; UEG GoogleMaps • 1 ☿; same collection data as for preceding; 1 Nov. 2010; UEG GoogleMaps • 1 ☿; same collection data as for preceding; 12 Nov. 2010; UEG GoogleMaps • 2 ☿☿; same collection data as for preceding; 8 Dec. 2010; UEG GoogleMaps • 2 ☿☿; same collection data as for preceding; 14 Dec. 2010; UEG GoogleMaps • 1 ☿; same collection data as for preceding; 21 Dec. 2010; UEG GoogleMaps • 4 ☿☿; same collection data as for preceding; 13 Jan. 2011; UEG GoogleMaps • 4 ☿☿; same collection data as for preceding; 20 Jan. 2011; UEG GoogleMaps • 3 ☿☿; same collection data as for preceding; 3 Feb. 2011; UEG GoogleMaps • 2 ☿☿; same collection data as for preceding; 17 Feb. 2011; UEG GoogleMaps • 1 ☿; same collection data as for preceding; 24 Feb. 2011; UEG GoogleMaps • 3 ☿☿; same collection data as for preceding; 17 Mar. 2011; UEG GoogleMaps • 1 ☿; Caiapônia, Cerradão ; 17°19′40″ S, 52°19′50″ W; 27 Jan. 2007; Vitor M. Carvalo leg.; Coleção Diniz; 8-9h A7; DZUP GoogleMaps • 2 ☿☿; same collection data as for preceding; 8-9h A9; DZUP GoogleMaps • 2 ☿☿; same collection data as for preceding; 15-16h A6; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 19-20h A7; DZUP GoogleMaps • 2 ☿☿; same collection data as for preceding; 10 Feb. 2007; 19-20h A9; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 24 Feb. 2007; 15-16h A6; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 15-16h A7; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 19-20h A6; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 10 Mar. 2007; 15-16h A8; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 19-20h A9; DZUP GoogleMaps • 1 ☿; Caiapônia , pasto ; 17°19′40″ S, 52°19′50″ W; 27 Jan. 2007; Vitor M. Carvalo leg.; Coleção Diniz, 15-16h A4; DZUP GoogleMaps • 1 ☿; Caldas Novas ; 1 Nov. 2011; H.L. Vasconcelos and T. Frizzo leg.; 952, T31-11S; DZUP • 1 ☿; same collection data as for preceding; 1020, T32-11S; DZUP • 1 ☿; same collection data as for preceding; 983, T33-17S; DZUP • 1 ☿; same collection data as for preceding; 1020, T32-17S; DZUP • 1 ♂; Campinas ; 1935; R. Spitz leg.; MZSP 62281 • 1 ☿; Chapada dos Veadeiros ; 13 Apr. 2015; J.B. Maravalhas leg.; 1088, T67-15S; DZUP • 1☿; same collection data as for preceding; 1187, T68-3S; DZUP • 1 ☿; same collection data as for preceding; 1187, T68-15S; DZUP • 1 ☿; Cocalzinho de Goiás , cerrado rupestre ; 15°47′43″ S, 48°49′45.76″ W; alt. 1321 m; 31 Jan. 2010; Eduardo de Oliveira Emery leg.; DZUP GoogleMaps • 2 ☿☿; Goiânia, campus Euc. De veterinária ; 23 Dec. 2001; Luciano Lozi leg.; Coleção Diniz; DZUP • 1 ☿; Goiás Velho ; 2 Jan. 1976; Guiflord leg.; MZSP • 1 ♂; Jataí ; Dec. 1972; F.M. Oliveira leg.; 8843; MZSP 62282 • 1 ☿; Mineiros ; 8 Feb. 2012; T. Frizzo leg.; 849, T38-11S; DZUP • 1 ☿; Mineiros , Parque Nacional das Emas ; 17°54′30.3″ S, 53°00′29.6″ W; 8 Feb. 2012; F. Camarota, T. Frizzo and R. Pacheco leg.; DZUP GoogleMaps • 1 ☿; Niquelândia, Anglo American ; 14°28′26″ S, 48°27′35″ W; 23 Jan. 2006; M. Vilela leg.; Coleção Diniz, IDSC (right) 19; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; INSC (right) 82; DZUP GoogleMaps • 2 ☿☿; same collection data as for preceding; INSC (left) 65; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; INSC (left) 23; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; IDSC (left) 77; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 22 Mar. 2006; IDSC (right) 88; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; 24 May 2006; INSC (right) 52; DZUP GoogleMaps • 1 ☿; Parque das Emas ; 11 Nov. 1990; C.R. Krigger leg.; UFSC • 1 ☿; P.E. Serra de Jaraguá ; 15.7964° S, 49.3335° W; 3 Jan. 2017; D.E. Oliveira leg.; DEO 843; DZUP GoogleMaps • 1 ☿; same collection data as for preceding; DZUP 549812 GoogleMaps • 1 ☿; P.N. Emas , Mineiros ; 25 Oct.–2 Nov. 1986; Bechara, Viviani, Ferro and Sanche leg.; MZSP • 1 ☿; Santa Bárbara ; 9 Dec. 2014; H.L. Vasconcelos and J.B. Maravalhas leg.; 643, T61-19S; DZUP • 1 ☿; same collection data as for preceding; 631, T63-11S; DZUP . – Mato Grosso • 1 ☿; Barra do Garça ; 6 May 2011; H.L. Vasconcelos and T. Frizzo leg.; 539, T11-7S; UFU • 1 ☿; Bonito ; 10 Jul. 2012; T. Frizzo leg.; 436, T43-11S; DZUP • 1 ☿; Buriti ; 15 Feb. 1967; N. Tangerini leg.; DZUP • 1 ☿; Cachoeira da Fumaça ; 9 May 2011; H.L. Vasconcelos and T. Frizzo leg.; 330, T13-3S; DZUP • 1 ♂; Chapada dos Guimarães; 23 Nov. 1983; Dep. Zool - UFPR (polonoroeste) exped.; DZUP • 1 ☿; same locality as for preceding; 24 Nov. 1983; Depto. Zool. (polonoroeste) exped.; DZUP • 1 ☿; same collection data as for preceding; 1–4 Feb. 1965; Sebastião Laroca leg.; DZUP • 1 ☿; same collection data as for preceding; 27 Jan. 1965; DZUP • 6 ☿☿; same collection data as for preceding; Nov. 1963; Alvarenga and Werner leg.; MZSP • 1 ☿; same collection data as for preceding; May 1959; Fr. Cannto leg.; MZSP • 4 ☿☿; Jan. 1960; C. Amann leg.; MZSP • 3 ☿☿; Jan. 1961; C. Amann leg.; MZSP • 1 ☿; 27 Oct. 1961; F.M. Oliveira leg.; DZUP • 1 ☿; same locality as for preceding; 15°27′11″ S, 55°44′23″ W; Oct. 2007; R. Silvestre et al. leg.; manual UFGD GoogleMaps • 1 ☿; Chapada dos Guimarães , Fazenda Buriti ; 18 Nov. 1982; Márcio Zanuto; MPEG HYM11513596 • 1 ☿; same collection data as for preceding; MPEG HYM11513598 • 1 ☿; Chapada dos Guimarães , Buriti ; Oct. 1972; G.R. Kloss and F. Val leg.; MZSP • 1 ♂; Itaum ; Mar. 1974; M. Alvarenga leg.; 10877; MZSP 62284 • 1 ♂; same data as for peceding; 10878; MZSP • 1 ♂; Maracaju ; Shannon Lane leg.; DZUP • 1 ☿; Nova Xavantina ; 10 May 2011; T. Frizzo leg.; 321, T16-7S; DZUP • 1 ☿; same collection data as for preceding; 311 T17-3S; DZUP • 1 ☿; PARNA Chapada dos Guimarães , Trilha do Cerrado ; 15°24′ S, 55°50′ W; alt. 617 m; Mar. 2016; R. Silvestre et al. leg.; UFGD GoogleMaps • 1☿; Vale dos Sonhos , Barra do Garças ; alt. 400 m; 22 Jun. 1972; Mielke and Brown leg.; DZUP 548841 • 1 ☿; Vale dos Sonhos , S. pr. B. do Garças ; 14 Jan. 1977; Kunze leg.; Coleção Diniz; 1394; DZUP • 1 ☿; Xavantina ; 28 Nov. 1949; W. Bollermann leg.; MZSP • 1 ☿; same locality as for preceding; 10 Jan. 1977; Kunze leg.; Coleção Diniz, 1392; DZUP . – Mato Grosso do Sul • 1 ☿; Bodoquena, Parque Nacional da Bodoquena , mata ciliar ; 10 Apr. 2008; T. Marques, J.H. Schoereder, R. Silvestre and interns leg.; arm 1 solo; UFV LabEcol 442 • 1 ☿; Bonito ; 9 Oct. 1989; Paiva R. leg.; MZSP • 1 ☿; Bonito, PARNA Serra da Bodoquena , Faz. Boqueirão ; 21°07′14.7″ S, 56°43′08.2″ W; 18–22 Dec. 2005; R. Silvestre et al. leg.; Hym 93-F; UFGD GoogleMaps • 1 ☿; Bonito, PARNA Serra da Bodoquena , Faz. Harmonia ; 21°17′09.8″ S, 56°41′45.5″ W; Oct. 2006; R. Silvestre et al. leg.; Hym 85F; UFGD GoogleMaps • 1 ☿; Bonito, RPPN Brazil Bonito , Rio Taquaral ; 21°06′27″ S, 56°38′14″ W; Nov. 2009; R. Silvestre et al. leg.; Hym 75-F; UFGD GoogleMaps • 1☿; Campo Grande ; 9 Oct. 1989; C.R.F. Brandão leg.; MZSP • 1 ☿; Campo Grande, Universidade Federal de Mato Grosso do Sul, RPPN UFMS ; 21 Oct. 2017; P.R. Souza leg.; ZUFMS 3064 • 1 ☿; Morraria do Sul, PN Serra da Bodoquena , Faz. Califórnia ; 20°42′07″ S, 56°52′47.7″ W; Nov. 2009; R. Silvestre et al. leg.; UFGD GoogleMaps • 1 ☿; Paranaíba , R. Paranaíba ; 14 Aug. 1972; em solo 506; Coleção Diniz; DZUP • 1 ☿; PARNA Serra da Bodoquena , Faz. Sta Laura da Vicunha , Rio Salobra ; 20°46′56.2″ S, 56°44′31.2″ W; 22–29 Jun. 2006; 3° exp., R. Silvestre et al. leg.; pitfall A-10, Hym 83-F; UFGD GoogleMaps • 1 ☿; P [Porto] Murtinho, Chaco Forest, Faz. Patolá ; 21°42′02″ S, 57°42′60″ W; 7 Mar. 2012; P.R. Souza et al. leg.; busca ativa; UFGD GoogleMaps • 1 ☿; same collection data as for preceding; DZUP 548837 GoogleMaps • 1 ☿; S do Paranaíba [Santana do Parnaíba], Fazenda Olho D′água ; 21 Feb. 1972; Diniz leg.; Coleção Diniz, 512, Em 9010; DZUP • 1 ☿; snt.do Paranaíba [Santana do Parnaíba], Faz. Olho D′Água ; 12 Jul. 1971; Diniz leg.; Coleção Diniz, 499, Em 9010; DZUP • 1 ☿; same locality as for preceding; 12 Jul. 1972; Diniz leg.; em solo; MZSP • 2 ☿☿; Serra da Bodoquena , Cara da Onça RPPN ; 20°44′24″ S, 56°44′11″ W; alt. 196 m; Dec. 2011; R. Silvestre leg.; manual; UFGD GoogleMaps • 1 ☿; Serra da Bodoquena , Faz. Campo Verde ; 21.3755° S, 56.7177° W; alt. 444 m; Dec. 2008; R. Silvestre leg.; manual; UFGD GoogleMaps • 2 ☿☿; Três Lagoas, Jardim Alvorada ; 3 Aug. 1985; J.L.M. Diniz leg.; Coleção Diniz, 2194; DZUP . – Minas Gerais • 1 ☿; Campo Florido ; 12 Feb. 1964; H.M. Canter leg.; MZSP • 1 ☿; Monte Carmelo ; 18.8000° S, 47.8000° W (3 min error); 23–25 May 2016; J.J.M. Aguiar leg.; amostra 355/176; ANTWEB 1038256 ; UFV GoogleMaps • 1 ☿; S do Salito [Serra do Salitre ]; 4 Apr. 1965; C. Elias leg.; DZUP • 1 ☿; Uberaba ; Oct. 1961; C. Elias leg.; DZUP • 1 ☿; Uberlândia ; Jan. 1999; W.L. de Melo leg.; DZUP 548840 ; DZUP . – Paraná • 1 ☿; C Mourão [Campo Mourão ]; Jul. 1950; coleção F. Justus; DZUP • 1 ☿; F do Iguaçu, Foz de Iguaçu cataratas ; 24 Aug. 2000; J.O. Schmidt leg.; MPEG • 1 ♂; Guarapuava ; Mar. 1947; 4380, Coleção F. Justus Jr; DZUP 548845 • 1 ☿; Guarauna ; Dec. 1940; coleção F. Justus, DZUP 548839 ; DZUP • 1 ☿; Laranjeira do Sul; Mar. 1947; coleção F. Justus, 4384; DZUP • 1 ♂; same locality as for preceding; Jan. 1962; S. Sakagami leg.; DZUP 548844 • 1 ☿; same collection data as for preceding; DZUP 548838 • 1 ☿; Palmas ; Dec. 1928; F. Schroer leg.; MZSP . – Santa Catarina • 1 ☿; Chapecó ; 2004; Funir leg.; P06; UFSC • 3 ☿☿; Nova Teutonia ; Fritz Plaumann leg.; MZSP • 1 ☿; same locality as for preceding; 30 Dec. 1940; Diringe leg.; MZSP • 1 ☿; same locality as for preceding; May 1957; Fritz Plaumann leg.; MZSP . – São Paulo • 1 ☿; Agudos ; May 1959; C. Gilbert leg.; 3524; MZSP • 3 ☿☿; same collection data as for preceding; Mar. 1960; MZSP • 1 ☿; Boa Esperança do Sul, Faz. Itaquerê ; 28 Nov. 1963; K. Lenko leg.; 2946; MZSP • 1 ☿; Botucatu ; 1 Sep. 1963; Mantovan leg.; MZSP • 1 ☿; same locality as for preceding; 18 Oct. 1968; M. Artamiro leg.; 6423; MZSP • 2 ☿☿; same locality as for preceding; 4 Dec. 1968; M. Artamiro leg.; 6422; MZSP • 1 ☿; Botucatu , CESP ; 15 Apr. 1991; B.H. Dietz leg.; MZSP • 12 ☿☿; Corumbataí ; 30 Dec. 1962; H.A. Britski leg.; MZSP • 1 ☿; Corumbataí, Fac. Fil. Rio Claro ; 1 Nov. 1963; DZUP • 1 ♂; Itirapina ; 10 Apr. 1990; R. Paiva leg.; MZSP 62283 • 2 ☿☿; same locality as for preceding; 27 Feb. 1968; D. Dias leg.; 4857; MZSP • 1 ☿; same locality as for preceding; 24 Oct. 1987; H.G. Fowler leg.; CPDC • 1 ☿; same locality as for preceding; 18 Nov. 1999; Thibaud Monnin leg.; MZSP • 1 ☿; Jundiaí ; Nov. 1929; J. Lane leg.; MZSP • 1 ☿; Rio Claro ; 1 Jul. 1962; S. Laroca leg.; DZUP • 1 ☿; same locality as for preceding; 18 Nov. 1983; Caetano and Brandão leg.; MZSP • 1 ☿; same locality as for preceding; Feb. 1992; E. Tomatake leg.; 4530; CPDC • 1 ☿; S [São] Carlos, Faz. Canchiu ; 9 Jun. 1987; R.R. Martins leg.; MZSP . – Rio Grande do Sul • 1 ☿; Derrubadas, Parque Estadual do Turvo , Floresta Semidecídua ; 25 Apr. 2009; T. Marques leg.; pitfall hipogeico; UFV LabEcol 441 • 1 ☿; Derrubadas, Parque Estadual do Turvo , mata seca ; 25 May 2009; T. Marques and interns leg.; arm 10 estrato sol; MZSP • 2 ☿☿; Sto [Santo] Ângelo ; 23 Feb. 1974; R.L. Araújo leg.; MZSP • 1 ☿; Santa Bárbara [Santa Bárbara do Sul] ; 23 Dec. 1944; C.R. Gonçalves leg.; MZSP • 2 ☿☿; Soledade, PPBio , Natural Grassland Ecossystem , campo ; 28.8725° S, 52.4588° W; Nov. 2013; L.R. Podgaiski leg.; manual collection; UFRGS GoogleMaps • 1 ☿; Tem [Tenente] Portela , P. Est. Rio Turvo ; Oct. 1978; E.P. Albuquerque leg.; MZSP • 1 ☿; Uruguaiana ; Aug. 2014; E. Gazbe leg.; MZSP . – Tocantins • 1 ☿; Paranã , cerrado sensu stricto ; 12.8140° S, 47.8980° W; 13 Oct. 2004; R.R. Silva and B.H. Dietz leg.; at night; MZSP GoogleMaps .
PARAGUAY – Amambaí • 1☿; Parque Nac. Cerro Corá ; 2 Nov. 1983; T. Bonace leg.; ibn0070; MNHNP HX68 • 1 ☿; same locality as for preceding; 24 Feb. 1981; ibn0066; MNHNP HX62 • 1 ☿; same locality as for preceding; 7–27 Mar. 1982; H. Ferreira da Cunha leg.; ibn0062; MNHNP HX66 • 1 ☿; same locality as for preceding; 5 Nov. 1983; H. Ferreira da Cunha leg.; MNHNP HX63 • 1 ☿; same collection data as for preceding; ibn0065; MNHNP HX64 • 1 ☿; same collection data as for preceding; ibn0063; MNHNP HX67 • 1 ☿; same locality as for preceding; 5 Jun. 1984; T. Bonace leg.; ibn0059; MNHNP HX65 • 1 ☿; same collection data as for preceding; ibn0067; MNHNP HX69 • 1 ☿; same locality as for preceding; 2 Nov. 1985; P. Muller leg.; MNHNP HX55 • 1 ☿; same collection data as for preceding; MNHNP HX56 • 1 ☿; same collection data as for preceding; MNHNP HX57 • 1 ☿; same collection data as for preceding; MNHNP HX58 • 1 ☿; PN Cerro Corá ; 12–16 Oct. 1981; J.A. Kolchaka leg.; MNHNP HX53 • 1 ☿; same collection data as for preceding; MNHNP HX54 • 1 ☿; same locality as for preceding; 22°38′19″ S, 56°01′12″ W; alt. 269 m; 3 Apr. 2015; R. Silvestre et al. leg.; UFGD GoogleMaps . – Itapúa • 1 ☿; Alto Verá ; 10 Nov. 1999; J.A. Kolchaka leg.; MNHNP HX59 • 1 ☿; same collection data as for preceding; MNHNP HX60 • 1 ☿; same collection data as for preceding; MNHNP HX61 • 1 ☿; Encarnación; n° 2646 coll Borgm.; MZSP • 1 ☿; same locality as for preceding; 2 Oct. 1898; C. Schrottky leg.; MZSP . – Misiones • 1 ☿; Santiago ; Oct. 1975; H. Fowler leg.; HFXXX, 13418; MZSP . – San Pedro • 1 ☿; Choré ; 25 Feb. 1988; Maria Noce de Meza leg.; MNHNP HX52 .
Redescription
Female
MEASUREMENTS. Lectotype: HL 5.1; HW 4.97; MDL 3.85; SL 4.78; MSL 7.36; HFL 6.4; HBL 5.6; PL 1.93; PH 2.99; PW 1.89; ATS 6.72; BL 24.97 (mm); CI 0.97; SI 0.96; DPI 0.97. Non-types (n = 61): HL 4.38–5.63; HW 4.04–5.35; MDL 3.05–4.28; SL 4.08–5.48; MSL 6.28–7.8; HFL 5.6–6.88; HBL 4.72– 5.9; PL 1.67–2.23; PH 2.48–3.2; PW 1.48–1.92; ATS 5.68–7.72; BL 22.06–27 (mm); CI 0.88–1.03; SI 0.85–1.07; DPI 0.8–1.
HEAD. Malar area with longitudinal to oblique striae that always reach anterior eye margin, sometimes extending to gena. Gena microareolate and opaque, sometimes silky, never smooth and shining, usually without rugulae. Apressed pubescence present between eye and frontal lobe, extending posteriorly to frons. Frons microareolate and opaque or with weak silky sheen; with flexuous, brownish and decumbent to suberect hairs, longer than scape diameter; pubescence short, appressed and scarce. Occipital corner varying from strongly microareolate and opaque to smooth and silky. Antennal scape microareolate and silky, suberect and long hairs usually present on antennal segments 1–3. Ventral surface of head microareolate with arched strigulae covering entire surface or just close to postgenal suture (rarely absent). Hypostomal tooth longitudinally strigulate. Labrum with median longitudinal sulcus weakly marked; with transverse rugulae. Mandibular dorsum weakly longitudinally strigulate on inner base, sculpture gradually fading apicad.
MESOSOMA. Dorsal margin of pronotum in lateral view broadly convex, usually with no pronounced dorsoposterior swelling; anteroventral corner of pronotum toothed or forming acute angle, rarely obtuse. Pronotal dorsum strongly microareolate and opaque to weakly microareolate and silky. Metapleuralpropodeal suture sinuous, with at least one curve ventral to position of propodeal spiracle.
METASOMA. Petiolar node in lateral view relatively short (DPI ≥ 0.8); anterodorsal corner at same level as posterodorsal corner, sometimes slightly lower; anterior margin straight or slightly concave, forming blunt angle with dorsal margin; dorsal and posterior margin each varying from broadly convex to straight and forming blunt to slightly rounded angle. Node lateral face usually microareolate and opaque, rarely slightly smooth and silky. Node anterior margin in dorsal view convex; posterior margin straight to broadly convex; lateral margins broadly convex and converging anterodorsally. Abdominal tergite III strongly microareolate and opaque to weakly microareolate and silky; punctulae in variable density, always denser laterally than dorsally; densely covered with brownish, flexuous, decumbent to suberect hairs on entire surface; appressed pubescence very sparse on dorsum, denser laterally.
Male
MEASUREMENTS. D. a. nigricolor lectotype: HL 2.07; HW1 2.3; MDL 0.56; SL 0.55; EL 1.19; MOD 0.31; LOD 0.33; MSL 5.93; HFL 4.6; PL1 1.34; PH 1.14; PW 1.12; ASL 3.25; BL1 13.16 (mm); CI1 1.1; SEI 2.14; SI1 0.24. D. a. bucki lectotype: HL 2.17; HW1 2.25; MDL 0.55; SL 0.62; EL 1.06; MOD 0.26; LOD 0.3; MSL 5.62; HFL 4.5; PL1 1.27; PH 1.09; PW 1; ASL 3.22; BL1 12.85 (mm); CI1 1.03; SEI 1.7; SI 10.27. Non-types (n = 7): HL 1.9–2.12; HW1 2.15–2.37; MDL 0.51–0.68; SL 0.46–0.68; EL 1.11–1.3; MOD 0.31–0.43; LOD 0.27–0.43; MSL 5.37–6.66; HFL 3.74–4.68; PL1 1.3–1.48; PH
1.14–1.42; PW 1.06–1.25; ASL 3.06–4.12; BL1 12.39–14.9 (mm); CI1 1.04–1.24; SEI 1.83–2.53; SI1 0.2–0.31.
HEAD. Frontal carina forming a short longitudinal line. Lateral ocellus usually reduced, sometimes not surpassing posterior head margin in full-face view. Head punctulate, very slightly microareolate and with silky sheen; with pallid decumbent pubescence and very few decumbent hairs only on clypeus and vertex. Antenna with very short appressed pubescence, without hairs. Ventral surface of head punctulate and slightly microareolate with silky sheen.
MESOSOMA. Mesoscutum without notaulus. Mesopleural sulcus usually mostly smooth. Scutoscutellar sulcus scrobiculate. Mesoscutellum usually longitudinally strigulate laterally to variable degrees. Metapleural-propodeal suture with same microsculpture as rest of integument. Mesosoma varying from microareolate and subopaque to smooth and shining, not becoming coarsely punctate on declivitous surface of propodeum; with short decumbent pubescence; without hairs. Legs densely covered by decumbent pubescence; without long hairs. Protibial apex usually without a stout seta.
METASOMA. Petiolar node usually slightly microareolate and with silky sheen; densely covered by decumbent to suberect pubescence, without hairs. Abdominal tergite VIII triangular, sometimes with a very sharp apex. Gaster usually microareolate and subopaque; tergites densely covered by appressed to decumbent pubescence; without long hairs.
GENITALIA. Basal ring in dorsal view with slightly concave lateral margins, anteriorly narrower than posteriorly; maximum diameter of fenestra longitudinally directed; median invagination usually V-shaped; in lateral view dorsal margin declining and extending anteriorly to form a broad lobe; anteroventral process subtriangular to trapezoid. Gonostylus broad and rounded. Dorsal margin of volsella in lateral view anteriorly straight to broadly convex and posteriorly concave; anteroventral corner projecting ventrally as a subtriangular, subquadrate or rounded lobe; rarely as a narrow and sharp process; posteroventral margin usually continuous, broadly concave and forming a discrete posterior rounded to acute lobe; digitus volsellaris with posterior margin straight to broadly convex. In lateral view, penisvalva with continuous dorsal and posterior margins, usually ending in a rounded apex which projects ventrolaterally; ventral margin uneven, usually with a concavity containing a short tooth or a spiniform projection; posteriorly with serrated rounded or triangular ventral lobe of variable size; anteroventral region concave or with a short triangular lobe.
COLOR. Body varying from completely light brown to black, sometimes with gaster much lightercolored.
Remarks
Dinoponera grandis , as discussed below, is strongly polymorphic. The paradox about this species is that females and males tell different histories when examined separately. While males of D. grandis will vary discretely in some characters, variation is more continuous in females. Because of this, even recognizing that D. grandis may represent a complex of several cryptic species, this is not tenable, based on the current information that separates these forms.
Females from southwestern Mato Grosso do Sul, Brazil, to Amambai, Paraguay, tend to have abdominal tergite III strongly microareolate and opaque and the anteroventral pronotal tooth long and acute, almost spiniform. Ants from Distrito Federal to the northeast of Goiás, Brazil, usually have the pronotal dorsum and abdominal tergite III weakly microareolate and with a silky sheen, and the anteroventral pronotal margin obtusely angular, edentate. Some specimens from southern Brazil have the pronotal dorsum and abdominal tergite III weakly microareolate and with a silky sheen. Some specimens from Paraná could be tentatively identified as D. lucida on account of their having the anterodorsal petiolar node angle slightly lower than the posterior angle, but in D. lucida this character is more pronounced plus the pubescence on the frons is abundant and long, quite different from D. grandis .
Besides the above-mentioned variation, all examined females of D. grandis have the malar striae reaching the eye, a relatively short petiolar node (DPI ≥ 0.8), a body length of less than 27 mm and a hind basitarsus less than 6 mm long. Occasionally, D. quadriceps and D. lucida may have some of these characters, but besides being usually larger, the following traits will also separate them. The petiolar node of D. lucida is anterodorsally lower than the posterodorsal corner and its abdominal tergite III is very smooth, with bluish iridescence. In D. quadriceps , the malar striae clearly fail to reach the eye and the petiolar node often has a higher anterodorsal corner.
Santschi (1928) described a form from Paraguay as Dinoponera grandis australis brevis based on female characters such as a scape length as long as the head length and a short petiolar node, one third higher than long. Borgmeier (1937) considered it as a subspecies, making the name D. australis brevis valid. However, Kempf (1971) did not consider these differences to be significant and he synonymized brevis under D. australis . Scape length in the studied specimens of D. grandis ranges from being longer to shorter than the head length. The node length was found to be one third higher than long in several specimens, leading to the conclusion that such differences are intraspecific.
Guérin-Méneville (1838) apparently described D. grandis based on two specimens. In the original description, the author referred to the extremely smooth and shining integument and to the petiolar node shape, which is rounded “up and forward”. These characters could refer to what is now considered D. longipes ; however, Guérin-Méneville also mentioned the type locality, probably in Minas Gerais, Brazil. Roger (1861) analyzed Guérin-Méneville’s specimens and recognized that one ant had somewhat sculptured and opaque integument, while the other was mostly smooth and shining. Roger also noted that one specimen had the petiolar node relatively narrower in lateral view, but despite this he synonymized D. grandis and D. gigantea . Dinoponera grandis , the more recent name, thus became a junior synonym of D. gigantea . In recent revisions, neither Kempf (1971) nor Lenhart et al. (2013) were able to study the types of these two species, so doubts lingered as to the status of D. grandis .
One of Guérin-Méneville’s syntypes in the ZSM was designated as the lectotype by Diller (1990). It corresponds to the form Roger described as opaque and with a short petiolar node, collected in Minas Gerais, Brazil.
Examining this type, we saw that it is definitely a different species than D. gigantea , its current synonym. It thus became obvious we had to separate these two species. Based on the type locality, it would be possible that D. grandis was a synonym of D. australis , D. lucida or D. quadriceps . These last two species, however, are easily separated from D. grandis . In addition, the lectotype of D. grandis was revealed to have the same characters used by Emery (1901) to describe D. grandis australis and later used by Borgmeier (1937) to raise D. australis to species level: body size smaller than in other species of Dinoponera ; anteroventral pronotal tooth present; integument less shining than in either D. longipes , D. lucida or D. mutica but more so than in D. gigantea ; short petiolar node, slightly longer than broad; short legs and antennae; and the scape surpassing the posterior head margin.
Due to the similarities between the lectotype of D. grandis and the description and the known forms of D. australis , allied with the fact that both occur in sympatry, these two species are synonymized. Dinoponera grandis , which is the oldest name, thus becomes the valid name (senior synonym) and D. australis becomes the junior synonym. Although the type of D. australis has not been examined, choosing not to synonymize these names would mean that there would be two names with the same diagnosis.
In this study three other names are synonymized under D. grandis : the species D. snellingi Lenhart, Dash & Mackay, 2013 , and the subspecies Dinoponera australis nigricolor Borgmeier, 1937 and Dinoponera australis bucki Borgmeier, 1937 . This decision is discussed below.
Borgmeier (1937) described D. australis nigricolor and D. australis bucki based on females and males taken from their respective nests, the former in central Goiás and the latter in Rio Grande do Sul, Brazil. He found D. a. nigricolor females to be identical to D. a. bucki females except for having the dorsal margin of the petiolar node margin slightly convex, a difference we consider as intraspecific. However, he considered male characters to be more reliable indicators, particularly gonostylus width. Other male differences he also considered diagnostic included the petiolar node shape, body color, shade of wing color and slight differences in the relative scape length. Unfortunately, he only had one male for each subspecies.
Lenhart et al. (2013) described D. snellingi based on 3 males collected at a light in Campo Grande, Mato Grosso do Sul, Brazil. The ants were initially identified as D. australis because they were collected at the same locality and on the same day as some females of D. australis . They compared these males with the descriptions by Kempf (1971) and found similarities in the short pygidial spine and bicolored body, but they also discerned a number of differences. They recognized it as a different species on account of the following combination of characters: bicolored body; head with bulging compound eyes and ocelli; penisvalva with a large ventral lobe and finger-like serrated flange; a short and broad digitus volsellaris with a finely toothed basal lobe; and a distinctively shaped paramere.
The present study was able to gather the largest number of D. grandis male specimens with the widest geographic coverage until now (11 male specimens, contrasting with five and four in Lenhart et al. 2013 and Kempf 1971, respectively). Below we discuss variation in the diagnostic characters given by Borgmeier (1937) and Lenhart et al. (2013), finding many of them to be ineffective for species delimitation and thus weakening the validity of these names.
Node shape, in general, is convex as seen laterally, but it can vary from evenly convex to unevenly convex, with the highest point shifting from mid-petiolar distance to points just anterior or posterior to it. Variability was found even in the relative height of the node. Given such variation, the differences in node shape used by Borgmeier to distinguish D. a. bucki from D. a. nigricolor becomes ineffective when more specimens are compared.
The male specimen of the D. a. bucki type series is designated here as the lectotype, given the apparent greater discreteness of male characters. It is strikingly bicolored with the gaster light chestnut and the rest of the body dark brown, but the bicolor pattern is also present in a male from Goiás (Jataí) and in another from São Paulo (Itirapina), the latter with a more yellowish gaster and darker body. Lenhart et al. (2013) described D. snellingi as having the same colors, but a male from Mato Grosso do Sul (Itaum), with genitalia very similar to those of D. snellingi , is entirely chestnut. The lectotype male of D. a. nigricolor is entirely black, with the gaster slightly lighter. This pattern was also found in males from Paraná (Guarapuava and Laranjeiras do Sul) and Mato Grosso do Sul (Itaum). Another male collected at the type locality of D. a. nigricolor is entirely light chestnut, as well as a specimen from Mato Grosso (Chapada dos Guimarães).
The relative scape length was found to be slightly less in D. nigricolor than in D. a. bucki by Borgmeier (1937). The ratio of scape width divided by scape length varies from 0.73 to 0.93 in six males, including the types, so this variation becomes muddled when studying more specimens.
In general, all males studied here have reduced eyes and ocelli compared with other species of Dinoponera , but only in the lectotype of D. a. bucki are they as small as those of the D. grandis male of fig. 4e in Lenhart et al. 2013. The lectotype of D. a. nigricolor , a topotypic male, and a specimen from Itirapina, São Paulo, all have the lateral ocelli surpassing the posterior head margin in dorsal view, as in the description of D. snellingi and fig. 4d in Lenhart et al. 2013. Therefore, this character is not exclusive to D. snellingi and its usefulness in separating species becomes doubtful.
Borgmeier (1937) found the gonostylus in D. a. nigricolor to be relatively wider than in D. a. bucki. Lenhart et al. (2013) described the gonostylus shape in D. snellingi as distinct from that in D. grandis . We detected differences in the shape and relative width of the gonostylus of other D. grandis males that are similar to those in D. a. bucki and D. a. nigricolor . Thus, these observed differences prove continuous among specimens, rendering them useless for diagnosis.
The anteroventral corner of the volsella in lateral view usually varies from rounded to subtriangular between specimens, with no evident pattern. An exception is provided by the lectotype of D. a. nigricolor and a topotype male, which have their anteroventral process narrow and sharp (spiniform). The posteroventral margin of the volsella of these specimens also differs by having a small subquadrate lobe close to the apex. All other males have a continuous margin. In the Itirapina male this margin is more strongly convex, giving the whole volsella an arched appearance.
All the observed males have a large anteroventral lobe on the penisvalva. The anteroventral corner, however, has two different states: either a concavity or a short triangular lobe. The latter state was found only in the Itaum and Itirapina males. The penisvalva illustration of D. snellingi in Lenhart et al. (2013) does not show this triangular lobe; instead it shows a more rounded and convex corner, which is also different from the concave corner found in other D. grandis males.
Penisvalva shape is the most variable character among examined males. In D. snellingi it is described by Lenhart et al. (2013) as having a large triangular ventral lobe with a vertical ridge running through the middle. This ventral lobe is longer than wide and is strikingly different from that of other Dinoponera males.Additionally, the ventral margin of the penisvalva has a small tooth. Males from Itaum and Itirapina have a very similar penisvalva shape, although the vertical ridge is inconspicuous. The penisvalva in the type of D. a. nigricolor and the topotypic specimen have a ventrally pronounced posteroventral lobe that forms a blunt angle, but it is never longer than wide, as in the illustration of the type of D. snellingi ( Lenhart et al. 2013: fig. 11b). The penisvalva of D. a. bucki has a rounded posteroventral lobe and a long, acute ventral spine, as do the other D. grandis males. It is very similar to the description of males from Argentina ( Lenhart et al. 2013).
Most of the characters mentioned above are not solid enough to justify the validity of D. snellingi , D. a. nigricolor or D. a. bucki. Even though the shape of the anteroventral corner in the volsella and penisvalva seems to vary discretely in D. a. nigricolor and D. snellingi , there are more gaps than certainties about the delimitation of these entities.
These males were taken during the same collection events as D. grandis females, but these few males do not allow us to know whether these seemingly distinct characters simply represent extremes of continuous variations.
A last consideration is that the validity of D. snellingi , D. a. nigricolor and D. a. bucki would imply we know the form of the D. grandis male, a tenuous assumption. The first description of a D. grandis male ( Santschi 1921: 85) was based on specimens from Argentina, very far from the type locality in Minas Gerais, Brazil. It is much more likely that the male of D. grandis corresponds to one of the specimens described as D. snellingi , D. a. nigricolor or D. a. bucki, all of which occur much closer to the type locality.
Based on all of this evidence, we argue for the synonymy of these names; however, we strongly encourage future studies on D. grandis , as the high variability of this species can be a sign of the existence of a complex of cryptic species. For future studies, we recommend that the first question addressed be: what is the form of the D. grandis male? The next step must be to gather fresh specimens by extensive fieldwork in all distribution areas, the results of which would also enable molecular and phylogeographic studies. In the light of human-induced climate change, and continuing deforestation and forest fires, we consider such fieldwork to be of utmost importance. Not only would it help elucidate the questions surrounding the situation of D. grandis , but it would also inform conservation measures that could help preserve the remaining populations of these giant ants.
Biology
Dinoponera grandis is found in a wide variety of environments, with predominance in savannas, at least for Brazilian records. Nests have a random spatial distribution and may have a density of up to 180 nests per hectare ( Tillberg et al. 2014). Each nest has an entrance without a surrounding mound and may reach a depth of 143 cm ( Paiva & Brandão 1995). Despite the relatively large nest size, colonies have on average only 14 workers, which indicates that nests are constantly reused and not completely constructed every generation ( Paiva & Brandão 1995; Monnin et al. 2003). Pheidole dinophila Wilson, 2003 has been recorded as nesting within nests of D. grandis at more than one locality ( Wilson 2003).
They are omnivorous, but favor hunting other invertebrates ( Tillberg et al. 2014). Foraging strategies involve route fidelity for individual workers and the distribution of different routes among workers, favoring greater efficiency in the area being covered ( Tillberg et al. 2014). Organization inside the nest reflects division of tasks, with foraging workers occupying chambers closer to the surface and higher status workers occupying deeper chambers ( Paiva & Brandão 1995). This nest occupation dynamic was confirmed by Smith et al. (2011), demonstrating that workers of deeper chambers have up to 39% of their dry body mass composed of fat, while workers found in shallow chambers may have less than 1% body fat. Dominance hierarchy is determined by agonistic conflicts similar to those in D. quadriceps , guaranteeing colony monogyny ( Monnin et al. 2003).
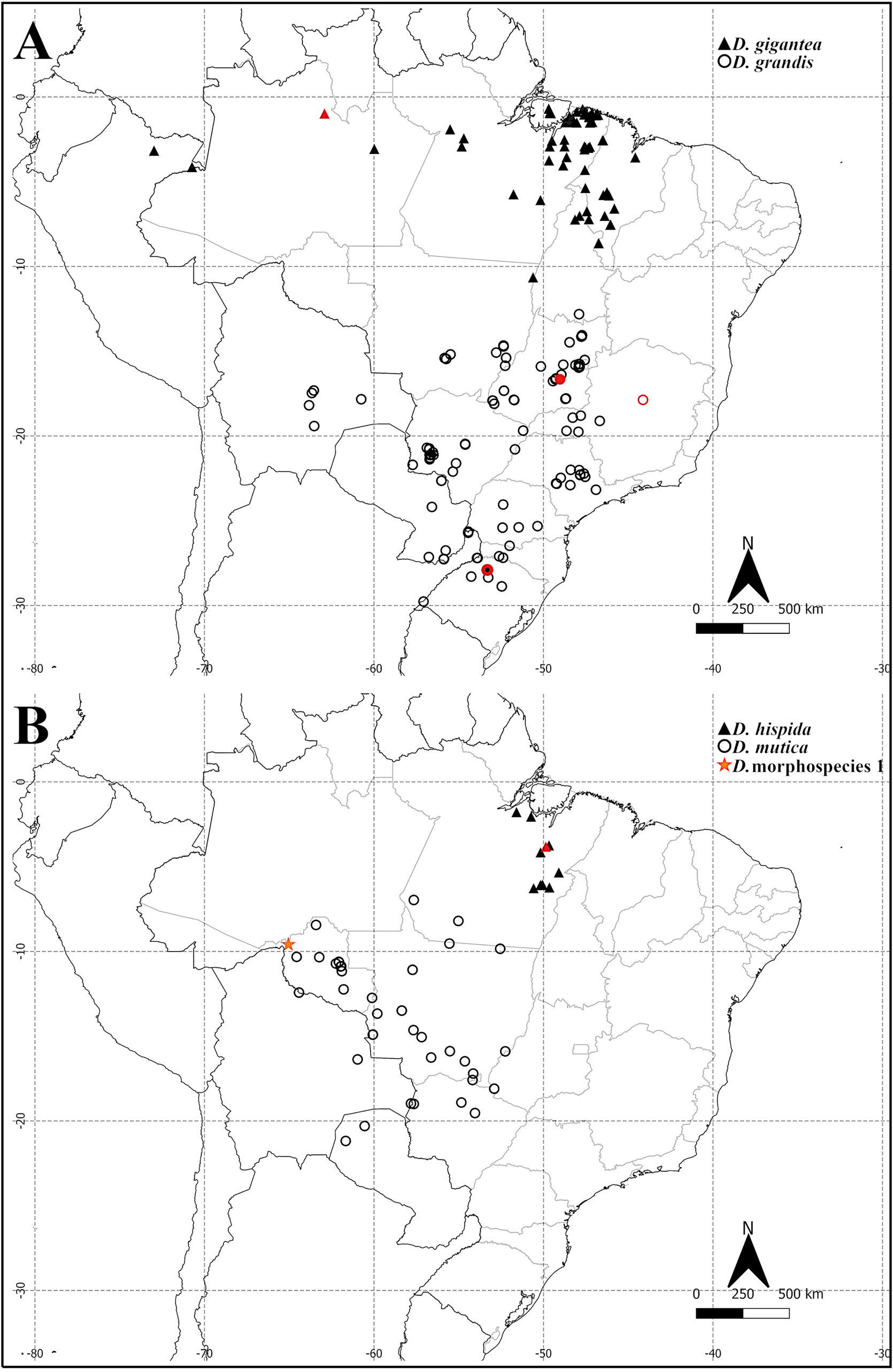
Distribution ( Fig. 28A View Fig )
Dinoponera grandis is a widely distributed species, occurring in most of the Central West, South and Southeast regions of Brazil as well as Paraguay, Argentina and Bolivia. The northernmost record is in Paranã (Tocantins) and the southernmost Brazilian record is in Uruguaiana (Rio Grande do Sul). These ants are not present in eastern Brazil.
Bequaert J. 1926. The date of publication of the Hymenoptera and Diptera described by Guerin in Duperrey's ' Voyage de La Coquille'. Entomologische Mitteilungen 15: 186 - 195.
Borgmeier T. 1937. Formigas novas ou pouco conhecidas da America do Sul e Central, principalmente do Brasil (Hym. Formicidae). Arquivos do Instituto de Biologia Vegetal 3 (2): 217 - 255.
Diller E. 1990. Die von Spix und Martius 1817 - 1820 in Brasilien gesammelten und von J. A. M. Perty 1833 bearbeiteten Hymenopteren in der Zoologischen Staatssammlung Munchen. Spixiana 13 (1): 61 - 81.
Emery C. 1901. Notes sur les sous-familles des Dorylines et Ponerines (famille des Formicides). Annales de la Societe entomologique de Belgique 45: 32 - 54.
Guerin-Meneville F. E. 1838. Premiere division. Crustaces, arachnides et insectes. In: Duperrey L. I. (ed.) Voyage autour du Monde, execute par Ordre du Roi, sur la Corvette de sa Majeste, La Coquille, pendant les Annees 1822, 1823, 1824 et 1825: 9 - 320. Arthus Bertrand, Paris.
Kempf W. W. 1971. A preliminary review of the ponerine ant genus Dinoponera Roger (Hymenoptera: Formicidae). Studia Entomologica 14: 369 - 394.
Kempf W. W. 1975. Miscellaneous studies on Neotropical ants. VI. (Hymenoptera, Formicidae). Studia Entomologica 18: 341 - 380.
Lenhart P. A., Dash S. T. & Mackay W. P. 2013. A revision of the giant Amazonian ants of the genus Dinoponera (Hymenoptera, Formicidae). Journal of Hymenoptera Research 31: 119 - 164. https: // doi. org / 10.3897 / jhr. 31.4335
Monnin T., Ratnieks F. L. W. & Brandao C. R. F. 2003. Reproductive conflict in animal societies: hierarchy length increases with colony size in queenless ponerine ants. Behavioral Ecology and Sociobiology 54: 71 - 79. https: // doi. org / 10.1007 / s 00265 - 003 - 0600 - 9
Paiva R. V. S. & Brandao C. R. F. 1995. Nests, worker population, and reproductive status of workers, in the giant queenless ponerine ant Dinoponera Roger (Hymenoptera Formicidae). Ethology Ecology & Evolution 7: 297 - 312. https: // doi. org / 10.1080 / 08927014.1995.9522938
Roger J. 1861. Die Ponera - artigen Ameisen (Schluss). Berliner Entomologische Zeitschrift 5: 1 - 54.
Santschi F. 1921. Ponerinae, Dorylinae et quelques autres formicides neotropiques. Bulletin de la Societe vaudoise des Sciences naturelles 54 (200): 81 - 103.
Santschi F. 1928. Sur quelques nouvelles fourmis du Bresil (Hym. Form.). Deutsche Entomologische Zeitschrift 1928: 414 - 416.
Smith C. R., Suarez A. V., Tsutsui N. D., Wittman S. E., Edmonds B., Freauff A. & Tillberg C. V. 2011. Nutritional asymmetries are related to division of labor in a queenless ant. PLoS One 6 (8): 1 - 5. https: // doi. org / 10.1371 / journal. pone. 0024011
Tillberg C. V, Edmonds B., Freauff A., Hanisch P. E., Paris C., Smith C. R., Tsutsui N. D., Wills B. D., Wittman S. E. & Suarez A. V. 2014. Foraging ecology of the tropical giant hunting ant Dinoponera australis (Hymenoptera: Formicidae) - evaluating mechanisms for high abundance. Biotropica 46 (2): 229 - 237. https: // doi. org / 10.1111 / btp. 12097
Tozetto L. & Lattke J. E. 2020. Revealing male genital morphology in the giant ant genus Dinoponera with geometric morphometrics. Arthropod Structure and Development 57: 1 - 12. https: // doi. org / 10.1016 / j. asd. 2020.100943
Wilson E. O. 2003. Pheidole in the New World. A Dominant, Hyperdiverse Ant Genus. Harvard University Press, Cambridge, MA.
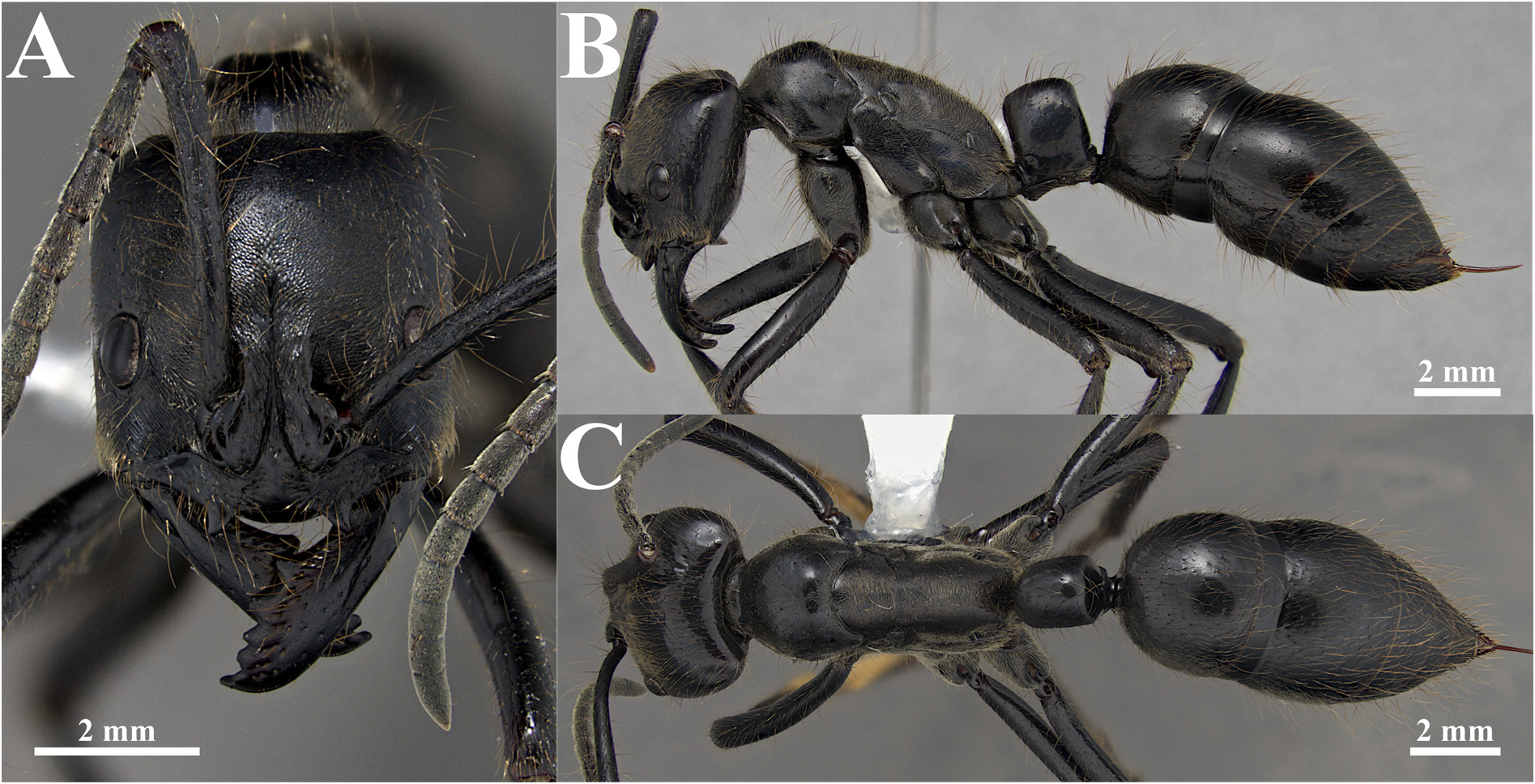
Fig. 15. Dinoponera grandis (Guérin-Méneville, 1838), ☿ (specimen from Jaraguá, Goiás, Brazil; DZUP549812). A. Full-face view. B. Full body in lateral view. C. Full body in dorsal view.
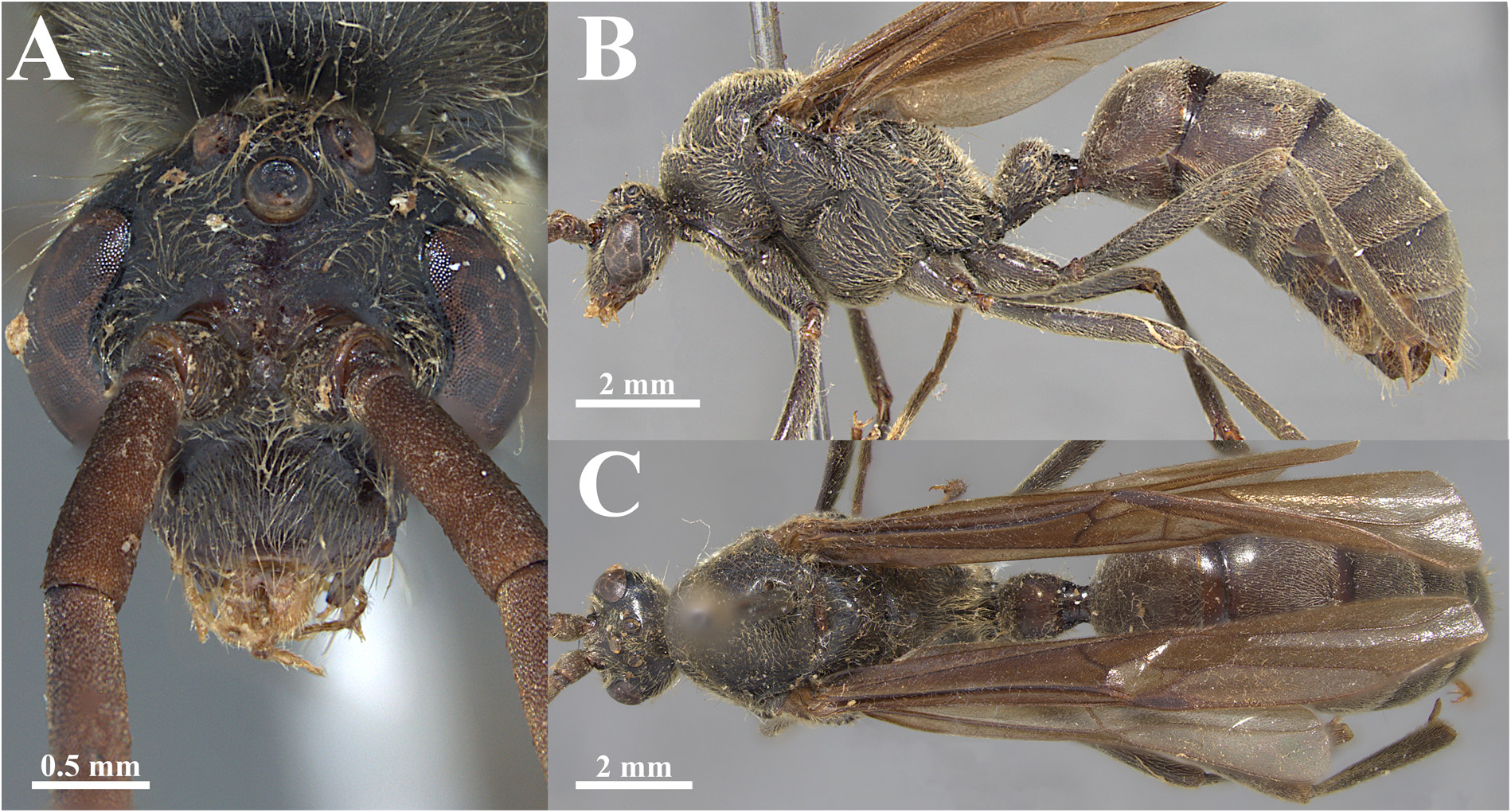
Fig. 16. Dinoponera grandis nigricolor Borgmeier, 1937 syn. nov., lectotype, ♂ (Campinas, Goiás, Brazil; MZSP 65801). A. Full-face view. B. Full body in lateral view. C. Full body in dorsal view.
Fig. 17. Dinoponera grandis bucki Borgmeier, 1937 syn. nov., lectotype, ♂ (Palmeiras das Missões, Rio Grande do Sul, Brazil; MZSP 65800). A. Full-face view. B. Full body in lateral view. C. Full body in dorsal view.
Fig. 18. Dinoponera grandis (Guérin-Méneville, 1838), ♂ (specimen from Itahum, Mato Grosso do Sul, Brazil; MZSP 62284). A. Full-face view. B. Full body in lateral view. C. Full body in dorsal view.
| ZSM |
Germany, Muenchen [= Munich], Zoologische Staatssammlung |
| MZSP |
Brazil, Sao Paulo, Sao Paulo, Museu de Zoologia da Universidade de Sao Paulo |
| INPA |
Brazil, Amazonas, Manaus, Instituto Nacional de Pesquisas da Amazoonia, Colecao Sistematica da Entomologia |
| CPDC |
Brazil, Bahia, Itabuna, Centro de Pesquisas do Cacau |
| DZUP |
Brazil, Parana, Curitiba, Universidade Federal do Parana, Museu de Entomologia Pe. Jesus Santiago Moure |
| MPEG |
Brazil, Para, Belem, Museu Paraense Emilio Goeldi |
| ZSM |
Bavarian State Collection of Zoology |
| UFRGS |
Universidade Federale do Rio Grande do Sul |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Ponerinae |
|
Genus |
Dinoponera grandis ( Guérin-Méneville, 1838 )
| Dias, Amanda Martins & Lattke, John Edwin 2021 |
Dinoponera australis brevis
| Kempf W. W. 1971: 382 |
Dinoponera australis nigricolor
| Kempf W. W. 1971: 387 |
Dinoponera australis brevis
| Borgmeier T. 1937: 227 |
Dinoponera australis bucki
| Borgmeier T. 1937: 228 |
Dinoponera australis nigricolor
| Borgmeier T. 1937: 228 |
Dinoponera grandis australis
| Santschi F. 1928: 416 |
Dinoponera grandis australis
| Tozetto L. & Lattke J. E. 2020: 5 |
| Lenhart P. A. & Dash S. T. & Mackay W. P. 2013: 135 |
| Kempf W. W. 1975: 382 |
| Borgmeier T. 1937: 227 |
| Santschi F. 1921: 85 |
Dinoponera grandis australis
| Emery C. 1901: 48 |
Dinoponera grandis
| Bequaert J. 1926: 188 |
| Roger J. 1861: 38 |
Ponera grandis Guérin-Méneville, 1838: 206
| Guerin-Meneville F. E. 1838: 206 |
1 (by felipe, 2021-12-22 14:00:53)
2 (by ExternalLinkService, 2021-12-22 14:08:38)
3 (by valdenar, 2021-12-22 16:04:37)
4 (by valdenar, 2021-12-22 17:26:54)
5 (by valdenar, 2021-12-22 18:25:18)
6 (by ExternalLinkService, 2021-12-22 18:35:29)
7 (by ExternalLinkService, 2021-12-22 19:07:21)
8 (by plazi, 2023-11-08 11:46:57)
9 (by ExternalLinkService, 2023-11-08 19:50:24)
10 (by ExternalLinkService, 2024-11-27 03:02:03)