Tubbia stewarti, Last, Peter R., Daley, Ross K. & Duhamel, Guy, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3616.5.3 |
|
publication LSID |
lsid:zoobank.org:pub:C7368437-2498-4ABB-B31A-664CA844A8EC |
|
DOI |
https://doi.org/10.5281/zenodo.5612584 |
|
persistent identifier |
https://treatment.plazi.org/id/EF64A3A4-7C1A-4FCB-B744-FF92C68C1232 |
|
taxon LSID |
lsid:zoobank.org:act:EF64A3A4-7C1A-4FCB-B744-FF92C68C1232 |
|
treatment provided by |
Plazi |
|
scientific name |
Tubbia stewarti |
| status |
sp. nov. |
Tubbia stewarti View in CoL sp. nov.
Seamount Rudderfish.
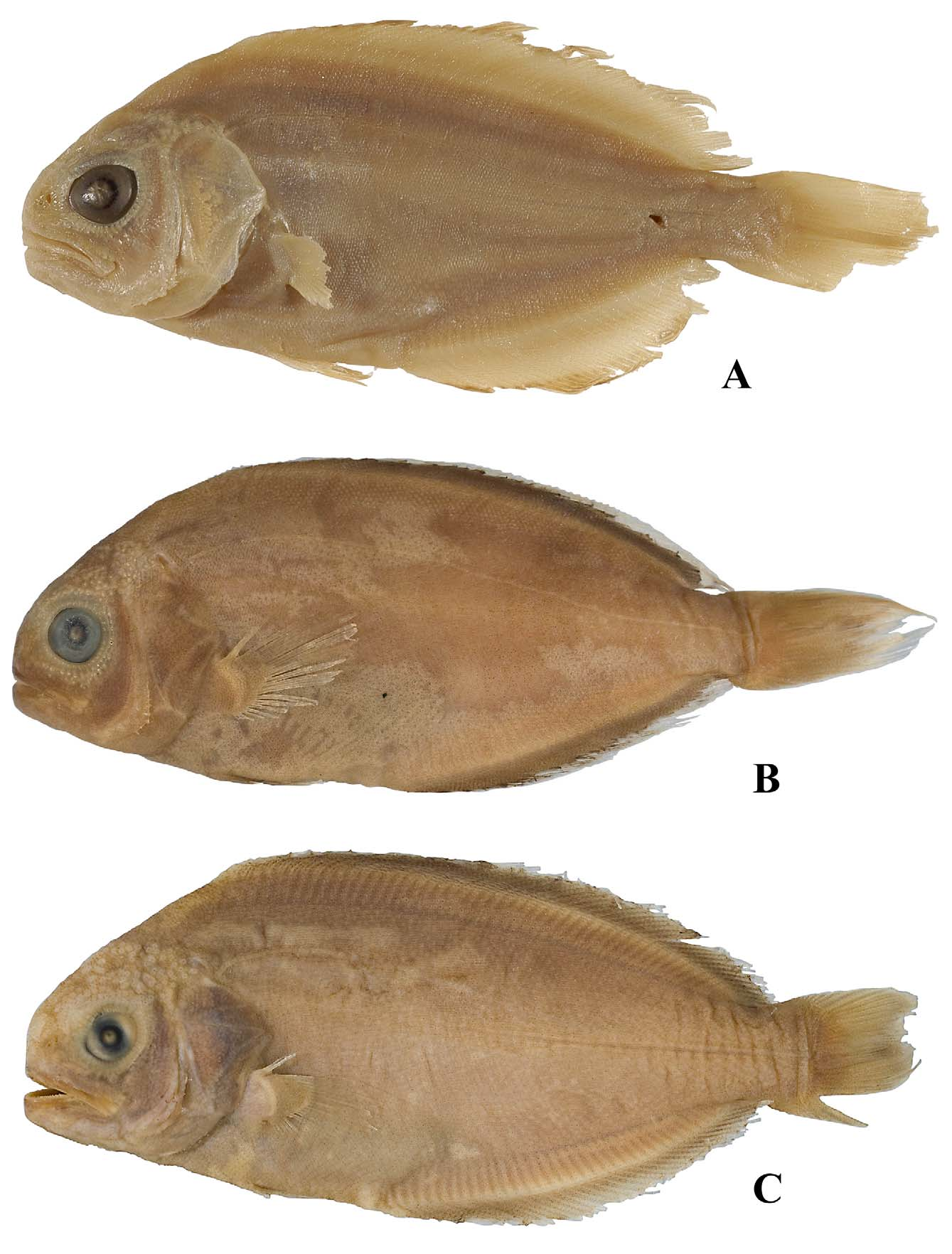
Figs 1 View FIGURE 1 A, 2A, 3, 5A; Tables 1 – 3 View TABLE 1 View TABLE 2 View TABLE 3
Tubbia tasmanica (not Whitley): McDowall, 1979, fig. 4 (misidentification). Tubbia sp. Daley et al., 1997, p 70, fig.
Holotype. NMNZ P. 0 33110, 434 mm SL, off Bay of Plenty, New Zealand, 37º 0 9.6′ S, 177º 0 7.98′ E, caught by commercial long line, depth not recorded, collected Gavin James, 1995.
Paratypes. 29 specimens. AMS I 45440 View Materials –001, 393 mm SL, Pedra Branca seamount, Tasmania, 44º12′ S, 147º10′ E, 650–775 m, 12 Nov 1992; AMS I 45441 View Materials –001, 368 mm SL, south Tasman Sea, Tasman Sea, 40º58′ S, 164º24′ E, 590–763 m, 19 Jun 2009; CSIRO A 4223, 61 mm SL, Storm Bay, Tasmania, 43º33′ S, 147º27′ E, 40–50 m, 8 Apr 1976; CSIRO H 2653–02, 110 mm SL, off King Island, Tasmania, 40º39′ S, 143º27′ E, 940–965 m, 7 Mar 1989; CSIRO H 2681–01, 88 mm SL, off Maatsuyker Island, Tasmania, Nov 1990; CSIRO H 3967–02, 405 mm SL, Pedra Branca seamount, Tasmania, 44º12′ S, 147º18′ E, 650–775 m, 12 Nov 1992; CSIRO H 3968–01, 401 mm SL, Pedra Branca, Tasmania, 44º20′ S, 147º22′ E, 705–725 m, 24 Nov 1992; CSIRO H 4372–02, 417 mm SL, Pedra Branca seamount, Tasmania, 44º11′ S, 147º10′ E, 525–650 m, 11 Apr 1993; CSIRO H 6880–02, 508 mm SL, south Tasman Sea, Tasman Sea, 40º36′ S, 162º08′ E, ca. 1014 m, 14 Jun 2008; CSIRO H 6966–03, 561 mm SL, St Helens seamount, Tasmania, 41º15′ S, 148º45′ E, 730–925 m, 18 Jul 2009; CSIRO H 6978–04, 490 mm SL, south Tasman Sea, Tasman Sea, 40º58′ S, 164º24′ E, 590–763 m, 19 Jun 2009; CSIRO H 7129–01, 400 mm SL, Pedra Branca seamount, Tasmania, 44º12′ S, 147º10′ E, 650–775 m, 12 Nov 1992; CSIRO T 42, 451 mm SL, off Bicheno, Tasmania, ca. 42º S, ca. 148º E, 1000 m, 26 Jul 1982; CSIRO T 74, 406 mm SL, off Bicheno, Tasmania, ca. 42º S, ca. 148º E, 1000 m, 26 Jul 1982; NMNZ P. 0 0 7612, 53 mm SL, Dusky Sound, New Zealand, 45º 45′ S, 166º 37′ E, 10 Feb 1973; NMNZ P. 0 0 9860, 364 mm SL, Chatham Rise, New Zealand, 42º 51′ S, 176º 44′ W, 880–894 m, 29 Jul 1980; NMNZ P, 0 11078, 99 mm SL, off North Cape, New Zealand, 34º 20′ S, 173º 16′ E, 30 m, 18 Nov 1978; NMNZ P, 0 11564, 499 mm SL, off Poor Knights Islands, New Zealand, 35º 14′ S, 175º 19’ E, 875–900 m, 22 Nov 1981; NMNZ P. 0 12893, 350 mm SL, Chatham Slope, New Zealand, 42º 37′ S, 176º 17′ E, 1065–1070 m, 28 Aug 1982; NMNZ P. 0 13381, 194 mm SL, Challenger Plateau, New Zealand, 41º 0 2′ S, 169º 30′ E, 908–910 m, 16 Feb 1983; NMNZ P. 0 25954, 367 mm SL, Challenger Plateau, New Zealand, 39º 40′ S, 167º 37′ E, 1080–1112 m, 27 Jul 1990; NMNZ P. 0 28727, 313 mm SL, East Chatham Rise, New Zealand, 43º 36′ S, 173º 52′ W, 1433–1438 m, 1 Jul 1992; NMNZ P. 0 31996, 110 mm SL, Chatham Rise, New Zealand, 42º 59′ S, 175º 49′ W, 778–780 m, 29 Feb 1992; NMNZ P. 0 37860, 182 mm SL, Solender Trough, New Zealand, 46º 48′ S, 166º 55′ E, 843–866 m, 13 Dec 2000; NMNZ P. 0 38753, 315 mm SL, Chatham Rise, New Zealand, 43º 10′ S, 173º 49′ W, 1060–1305 m, 9 Feb 2003; NMNZ P. 0 40877, 320 mm SL, Chatham Rise, New Zealand, 43º 36′ S, 174º 16′ W, 890–896 m, 23 Jul 2004; NMNZ P. 0 45146, 502 mm SL, Challenger Plateau, Tasman Sea, 39º 50′ S, 168º 0 3′ E, 864–886 m, 27 Jun 2005; NMV A 26357–001, 408 mm SL, Pedra Branca seamount, Tasmania, 44º12′ S, 147º13′ E, 525–650 m, 12 Nov 1992; NMV A 26358–001, 429 mm SL, south Tasman Sea, Tasman Sea, 40º57′ S, 164º25′ E, 763–912 m, 19 Jun 2009.
Diagnosis. A species of Tubbia with the following combination of characters: head of adult robust with a relatively broad and moderately arched interorbit (its width 7.8–8.9% SL in specimens exceeding 300 mm SL); nostrils large, width of posterior nostril 10–12 times in postorbital head length in adults (exceeding 300 mm SL); eye relatively large in adults (diameter 5.9–7.0% SL) and close to dorsal margin of head (closest horizontal distance 2.0–3.2 times in diameter of orbit); jaws relatively large, head length 2.2–2.5 times length of lower jaw; head pores very dense; caudal peduncle relatively narrow at insertion of anal fin, width 2.4–3.0% SL in juveniles (less than 110 mm SL), 3.3–5.2% SL in adults (exceeding 300 mm SL); vertebral centra 44–45.
Description. Proportional measurements of the types are given in Table 1 View TABLE 1 with specific ratios provided below. Data for the holotype are presented first; ranges for paratypes are given in parentheses (juveniles 61–110 mm SL firstly, followed by adults 313–499 mm SL).
Dorsal-fin elements 50 (48–53); anal-fin elements 34 (32–38); pectoral-fin rays 20 (21–23; caudal-fin principal rays 9 (9) + 8 (8) = 17 (17), upper procurrent rays 9–13, lower procurrent rays 9–13; total vertebral centra 44–45.
Body elongate oval-shaped; strongly compressed, more so posteriorly; greatest depth from mid-abdomen to vent; anterior head at snout tip almost vertical in lateral view, its margin moderately convex (usually elevated to hind orbit, then becoming less so to dorsal-fin origin); abdomen weakly convex (usually extended slightly); profile at bases of dorsal and anal fins convex, tapering on tail at caudal peduncle; caudal peduncle relatively deep (almost uniform in depth anteriorly and posteriorly), strongly compressed; procurrent rays of caudal fin forming keel-like expansions on dorsal and ventral edges of tail.
Head relatively large, deep, length 27.6% (29.7–35.5% and 23.7–28.3% SL in juveniles and adults respectively); moderately compressed and robust, width 43% (34–45%, 44–52%) of length, slightly to much broader than trunk. Snout short, blunt, its horizontal length 19% (16–22%, 16–21%) of head length. Nostrils relatively enlarged, openings closely adjacent, positioned slightly forward of or at midpoint of snout and mostly below level of mid eye; anterior opening simple, subcircular, tubular; posterior opening narrow, more slit-like, subvertical, its length usually exceeding aperture of anterior nostril. Eye relatively large, 1.3 (1.2–2.0, 1.1–1.7) times horizontal snout length, 4.2 (3.0–4.0, 3.6–4.2) in head length; lateral on head, not protruding but marginally visible when viewed front on; relatively close to dorsal surface, shortest horizontal distance from eye to dorsal margin of head 3.2 (1.7–4.4, 2.0–2.9) in eye diameter; interorbital space relatively broad, width 1.2 (1.0–1.2, 1.2–1.4) in eye diameter; not strongly arched; weak to well-developed supraorbital crest present.
Mouth large, slightly oblique, length of lower jaw 2.4 (2.1–2.3, 2.2–2.5) in head length; not protrusible, subterminal to slightly inferior; lips narrow, firm; maxilla elongate, slender, extending almost to hind margin of eye; gape narrow, 1.5 (1.5–2.2, 1.2–1.5) in length of maxilla; lower jaw partly enclosed within upper jaw when mouth closed; a tall, bony, chisel-edged ridge along each jaw bearing small, villiform teeth. Teeth similar in size and shape; uniserial around entire length of both jaws (including premaxilla and dentary); absent from vomer and palatines; roof of mouth and tongue covered with numerous fine, tooth-like papillae. Inner anterior margins of mandibles not united, separated by triangular fleshy anterior portion of branchial arch.
Gill openings extensive, opercular flap well developed, postorbital length 1.5 (1.5–2.1, 2.0–2.3) times eye diameter. Opercular margin irregular with fine soft spines and papillae; variably developed, soft, flexible, thallate spine at posterior extremity, sometimes with an obscure smaller spine above. Preopercular margin irregular, finely spinose and papillate; anteriormost part posteroventral to angle of jaw; posteroventral extremity narrowly rounded (following contour of operculum); upper part oblique, directed anterodorsally, terminating just above horizontal level of mid eye. Opercular membranes not united, free from isthmus. Gill rakers on first arch relatively large, elongate, 4–7+1+13 (n=6); on lower limb decreasing in length anteriorly (ca. 13 mm to 4 mm in length) from angle; sickle shaped, bases of longest rakers stout, tapering strongly to a point distally; posterior inner margins with fine hair-like spines.
Dorsal fin with 48–53 total elements; single, long, low, continuous; anteriorly with 4–6 short, soft spines followed by 43–47 rays, usually obscured, origin indistinct, over hind margin of operculum; insertion more or less over anal-fin insertion; base indistinct, embedded and merging with body; soft spines grading anteriorly into longer rays; all elements raked posteriorly, even when fin raised; tips of posterior rays well short of caudal fin when depressed; anterior rays simple or weakly branched (sometimes narrowly divided distally); rays 16–21 longest, subequal to or shorter than pectoral fin. Anal fin similar in shape to dorsal fin, with 32–38 total elements; base obscured with 2–3 short, soft spines followed by 31–36 rays; posterior rays falling well short of caudal-fin base when depressed; rays 1–2 reduced; rays 7–11 longest, subequal to longest rays of dorsal fin. Pectoral fin short, with 20–21 rays; paddle-shaped to rounded, base relatively broad, subvertical to weakly oblique, situated below level of eye; rays mostly weakly branched, lowermost rays fully or partly concealed under a scaly sheath of skin. Pelvic fins greatly reduced in adult, with 1 flexible spine and 5 rays; bases closely adjacent and situated below pectoral-fin bases, depressible into shallow groove on anteroventral midline of abdomen. Caudal fin with 17 primary elements (9 upper, 8 lower), 19–26 shorter procurrent rays; fin well developed, broad based, forked (appearing emarginate when damaged), with narrowly rounded tips; caudal peduncle deeper than long, depth of body at vent 3.1 (3.3–4.6, 3.2–3.9) times minimum depth of caudal peduncle.
Body and fins sheathed in thick skin covered with small deciduous scales (intact in holotype but variably abraded in most paratypes). Scales cycloid, weakly imbricated, variable in shape; absent on orbital membrane, nostrils, lips and on sensory pores (scales adjacent sensory pores usually modified greatly in shape to fit around pore); their size variable, largest on flanks, expanded longitudinally on dorsal and anal fins, very small on caudal, pectoral and pelvic fins. Skin on flanks and over unpaired fins thick; densely covered with indistinct longitudinal rows of small, weakly or non-emergent pores; pores closely and almost equidistantly spaced; externally, more prominent beside bases of dorsal and anal fins than on central flank; pores more evident on undersurface of skin than on external surface; papillose fibres connect skin to musculature on sides, distal edges broken when skin removed ( Fig. 6 View FIGURE 6 ). Pale pores most prominent on head, slightly elevated; particularly prominent on snout, around orbit, and on lower jaw; pores on anterior nape and upper snout somewhat reticulate in appearance, merging with regular predorsal scaled patch on midline of nape above hind margin of eye. Lateral line usually distinct, slightly undulatory, with ca. 130 pored scales in holotype (ca. 139 in CSIRO H 3968–01); arched weakly anteriorly, following contour of dorsal surface to caudal-fin base; scales modified slightly but barely distinct from those adjacent.
Ontogenetic variation. Like most other members of the family, juveniles differ markedly from adults in morphology and colour. Also, as adults and juveniles are soft-bodied, considerable intraspecific variability in morphometrics might be expected. Ontogenetic differences were most evident in measurements about the head, from the snout to the dorsal and anal-fin origins, distance between fin bases, and pectoral and pelvic-fin lengths. In summary, juveniles (less than 110 mm SL) have a relatively shorter pectoral-anal length (20.3–20.9% vs. 22.3–23.8% SL), caudal peduncle width at anal-fin insertion (2.8–3.0% vs. 3.3–5.2% SL) and caudal peduncle (1.9–4.7% vs. 6.1–8.7% SL), and a larger predorsal length (33.5–36.3% vs. 25.7–28.8% SL), preanal length (57.3–62.3% vs. 50.0–51.1% SL), head length (29.7–35.5% vs. 23.7–28.3% SL) and scaleless part of head (17.7–21.0% vs. 13.1–16.4% SL), postorbital head (15.7–17.6% vs. 12.9–14.6% SL), snout length (7.2–8.0% vs. 4.5–6.5% SL), eye diameter (8.1–12.4% vs. 5.9–7.0% SL), anterior (1.4–1.8% vs. 0.7–0.8% SL) and posterior nostril (2.2–3.0% vs. 1.1–1.3% SL) widths, upper-jaw (14.4–16.6% vs. 11.4–12.6% SL) and lower-jaw (13.9–15.7% vs. 10.7–11.5% SL) lengths, prepectoral (30.7–35.5% vs. 23.7–26.3% SL) and prepelvic (30.2–33.8% vs. 25.8–27.1% SL) lengths, pectoral (17.3–20.6% vs. 9.7–12.3% SL) and pelvic-fin lengths (14.5–20.3% vs. 5.0–6.8% SL), and a deeper head (at isthmus 19.7–21.6% vs. 17.4–17.8% SL) and body (at vent 37.6–41.2% vs. 30.1–34.2% SL), than adults (exceeding 313 mm SL).
Coloration. When fresh (holotype): Almost uniformly dark chocolate brown, eyes bluish; similar in preservative with pale eyes. In preservative (paratypes): similar to holotype but with extensive irregular paler patches on sides, particularly on the head (scale pockets demarcated when scales removed); orbital rim, branchiostegal membrane, lips, and nostrils often noticeably darker than rest of head; eye mostly pale, with dark outer margin coincident with rim of orbit.
Etymology. Named in honour of New Zealand ichthyologist, Andrew Stewart, whose efforts in building a substantial collection of stromateoid fishes from the region has contributed so significantly to our understanding of the life histories and composition of this poorly known group of fishes in the Southern Hemisphere.
Size. Reaches at least 561 mm SL; smallest available specimen 53 mm SL.
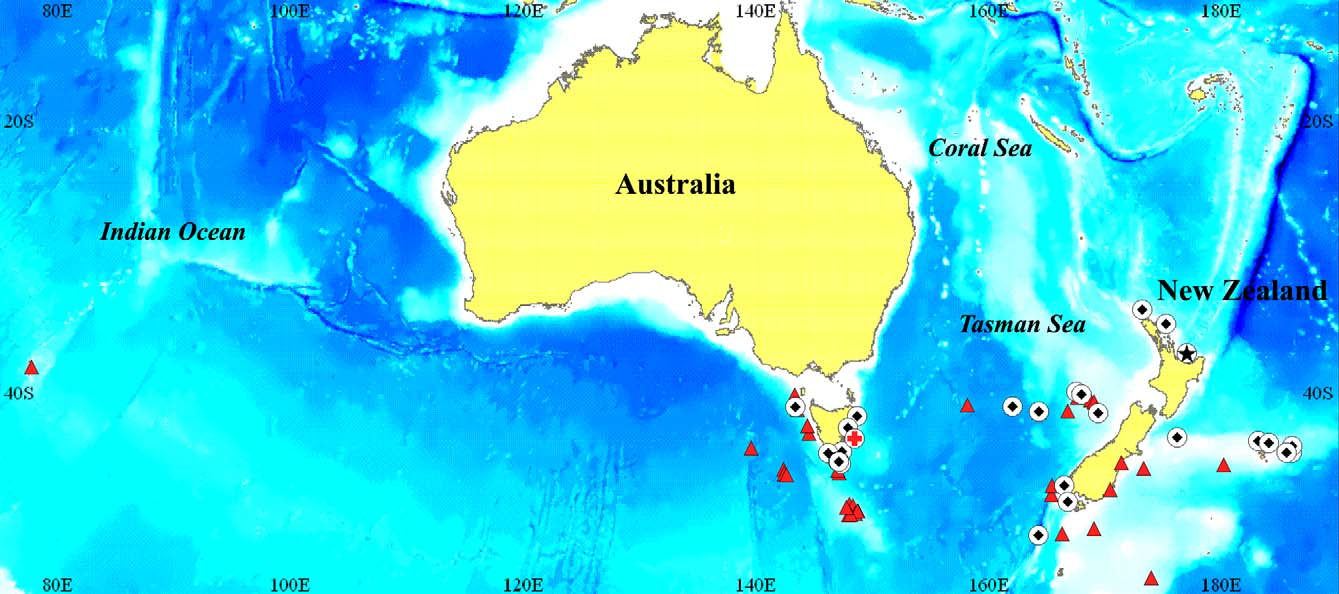
Distribution. Confirmed from off Australia and New Zealand in the Southern Hemisphere ( Fig. 7 View FIGURE 7 ) with most material collected from deep offshore plateaus and seamounts. Possibly more widespread in the southern Indian Ocean but this needs confirmation. Juveniles (61–99 mm SL) collected from the epipelagic zone at 30–50 m depth; larger individuals (110–561 mm SL) occur much deeper in the mesopelagic zone at 525–1438 m depth.
Comparisons. Tubbia stewarti differs subtly from T. tasmanica in morphology and meristics and the two species have been distinguished by their muscle protein characteristics as well as barcoding using the CO1 gene ( Fig. 8 View FIGURE 8 ). Adult T. stewarti have a relatively broader head with a flatter, less well arched interorbit (width 7.8–8.9% vs. 6.4–7.9% SL in specimens exceeding 300 mm SL), larger nostrils (width of posterior nostril 5–7 vs. 12–17 times in postorbital head length in juveniles less than 110 mm SL, 10–12 vs. 14–26 times in adults exceeding 300 mm SL), larger eye (diameter 5.9–7.0% vs. 5.2–6.3% SL), longer jaws (head length 2.2–2.5 vs. 2.5–2.8 times length of lower jaw), narrower caudal peduncle (width at insertion of anal fin 2.4–3.0% vs. 3.5–4.7% SL in juveniles less than 110 mm SL, 3.3–5.2% vs. 5.3–6.1% SL in adults exceeding 300 mm SL), and more vertebral centra (44–45 vs. 40–43). Tubbia stewarti appears to reach a slightly larger size than T. tasmanica (known to reach 561 mm SL vs. 407 mm SL) and is typically more elongate as an adult (depth at vent 29.4–34.2% vs. 32.3–38.1% SL). The eye of T. stewarti is also closer to the dorsal surface of the head (closest horizontal distance from eye to head margin 1.7–4.4 times vs. 1.0–1.6 times eye diameter in juveniles, 2.0–3.2 times vs. 1.5–1.9 times eye diameter in adults of T. tasmanica ). Head pores are much more densely distributed in T. stewarti than T. tasmanica and this state is most pronounced around the eye and on the lower jaw (sees Fig. 5 View FIGURE 5 ). Tubbia stewarti has typically more dorsal-fin elements but a similar anal-fin count ( Table 3 View TABLE 3 ).
Remarks. Published data for T. tasmanica also inadvertently contains data for T. stewarti . A 57 (now 53) mm SL specimen (NMNZ P.007612) figured by McDowall (1979, p 737, Fig. 4 View FIGURE 4 ) as T. tasmanica was re-examined in this study and found to be a juvenile of T. stewarti . McDowall’s vertebral count and selected morphometrics for this specimen are consistent with the diagnosis of T. stewarti . Also, a poorly preserved specimen ( RUSI 7423) reported as T. tasmanica in the same paper from off Natal (western Indian Ocean) but not examined in this study, has a documented vertebral count typical of T. stewarti (i.e. of 44). This specimen might constitute the first record of the species from the Indian Ocean.
The natural histories of Tubbia species are not well understood. The young appear to live mainly in the epipelagic zone, probably in association with jellyfish. A collection of 11 juvenile Tubbia tasmanica (CSIRO H 5994–01, 35.6–85.6 mm SL), obtained from the gut content of a shortsnout lancetfish ( Alepisaurus brevirostris Gibbs ) caught in the open ocean off southwestern Tasmania in December 1992, suggest these fishes may be locally abundant at some periods. Adult fish, consistent with many other centrolophids, appear to descend to the meso- and bathypelagic zones where they have been caught at almost 1500 m depth. Tubbia are oily compared to most other Australian fishes, and also extremely unusual in having high levels in the flesh of squalene, a liver oil present in deepsea sharks (Nichols et al, 2001). While these oils may play a similar role in buoyancy control, their exact role is unknown.
The chisel-edged jaws of Tubbia species are lined with fine teeth adapted to firstly grasping and then slicing through the soft and flexible tissues of pelagic invertebrates, such as coelenterates and ctenophores. The lower jaw, which internally overlaps the upper jaw, probably uses a scissor-like action to cleanly remove lumps of tissue. This feeding action is likely to be possessed by other centrolophid taxa. Tubbia stewarti has larger maximum size, and larger mouth and gill rakers than T. tasmanica , and probably feeds on larger prey species.
TABLE 1. Comparative morphometrics of adult Tubbia stewarti sp. nov., based on the holotype (NMNZ P. 0 33110, 434 mm SL) and ranges for five paratypes (313 – 499 mm SL), and seven adult specimens of T. tasmanica (302 – 407 mm SL). Morphometric characters are expressed as percentages of standard length (SL).
| Tubbia stewarti | Tubbia tasmanica | ||
|---|---|---|---|
| Holotype Min | Max | Min Max | |
| Standard length (mm) | 434 313 | 499 | 302 407 |
| Caudal fork length | 114.7 109.2 | 113.7 | 111.2 114.9 |
| Predorsal length | 28.5 25.7 | 28.8 | 22.3 27.3 |
| Preanal length | 53.0 50.0 | 51.1 | 45.1 51.7 |
| Prepectoral length | 27.1 23.7 | 26.3 | 23.3 26.3 |
| Prepelvic length | 28.3 25.8 | 27.1 | 25.5 28.1 |
| Pectoral-anal length | 22.7 22.3 | 23.8 | 20.3 25.0 |
| Pectoral-pelvic length | 7.6 7.6 | 10.9 | 6.3 8.2 |
| Pelvic-anal length | 24.9 23.8 | 26.5 | 20.6 25.5 |
| Head length | 27.6 23.7 | 28.3 | 23.5 28.7 |
| Snout length (horizontal) | 5.2 3.8 | 5.7 | 3.4 5.3 |
| Snout length (direct) | 6.4 4.5 | 6.5 | 5.3 6.4 |
| Postorbital head length | 15.1 12.9 | 14.6 | 13.4 17.4 |
| Length of naked part of dorsal head | 16.1 13.1 | 16.4 | 11.0 14.3 |
| Interorbital width | 8.1 7.8 | 8.9 | 6.4 7.9 |
| Eye diameter | 6.6 5.9 | 7.0 | 5.2 6.3 |
| Distance eye-top of head (horizontal) | 2.1 2.3 | 3.1 | 2.8 3.8 |
| Anterior nostril width | 0.6 0.7 | 0.8 | 0.3 0.8 |
| Posterior nostril width | 1.3 1.1 | 1.3 | 0.5 1.0 |
| Upper-jaw length | 12.8 11.4 | 12.6 | 9.6 11.3 |
| Lower-jaw length | 11.5 10.7 | 11.5 | 8.9 10.3 |
| Gape width | 8.6 7.7 | 9.3 | 7.6 9.1 |
| Dorsal-fin base length | 67.8 63.1 | 68.0 | 65.8 70.7 |
| Anal-fin base length | 40.3 40.0 | 43.5 | 40.8 47.1 |
| Pectoral-fin length | 10.5 9.7 | 12.3 | 9.5 11.4 |
| Pelvic-fin length | 4.8 5.0 | 6.8 | 4.8 6.4 |
| Head depth (at isthmus) | 14.9 17.4 | 17.8 | 15.1 19.1 |
| Head width | 11.8 11.0 | 13.9 | 10.7 14.0 |
| Body depth (greatest) Body depth (at vent) | 30.1 31.3 29.4 30.1 | 34.3 34.2 | 33.3 39.3 32.3 38.1 |
| Body width (across trunk) | 11.6 8.8 | 13.4 | 10.2 14.3 |
| Caudal peduncle depth (minimum) | 9.3 8.8 | 9.7 | 9.1 10.5 |
| Caudal peduncle length | 6.2 6.1 | 8.7 | 5.6 8.1 |
| Caudal peduncle depth (at anal insertion) | 10.2 10.1 | 10.9 | 10.0 10.9 |
| Caudal peduncle width (at anal insertion) | 5.1 3.3 | 5.2 | 5.3 6.1 |
TABLE 3. Comparison of primary meristic data for Tubbia stewarti sp. nov. and T. tasmanica: A, dorsal-fin; B, anal-fin; and C, total vertebrae counts.
| A. Dorsal-fin elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | N | |
| Tubbia stewarti | 6 | 5 | 8 | 5 | 2 | 2 | 28 | ||
| Tubbia tasmanica | 3 | 3 | 9 | 8 | 5 | 28 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |