Chaetocirratulus gayheadius ( Hartman, 1965 ), 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5113.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:EB01C862-025E-493F-8CA9-934B4F1626AF |
|
DOI |
https://doi.org/10.5281/zenodo.6958002 |
|
persistent identifier |
https://treatment.plazi.org/id/054C717B-7112-2371-65DD-FEEDFD1DFC90 |
|
treatment provided by |
Plazi |
|
scientific name |
Chaetocirratulus gayheadius ( Hartman, 1965 ) |
| status |
comb. nov. |
Chaetocirratulus gayheadius ( Hartman, 1965) new combination
Figures 3–7 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7
Table 1 View TABLE 1
Chaetozone gayheadia Hartman, 1965: 166 View in CoL ; Hartman & Fauchald 1971: 109–110 (in part); Maciolek et al. 1987b: D-2 (in part).
Cirratulus gayheadius: Petersen 1991: 592, 1999: 112 , 117, Fig. 3A–D View FIGURE 3 .
Chaetocirratulus gayheadius: Blake 2018: 121 .
Material examined. ( 96 specimens) Off New England, south of Gay Head , Martha’s Vineyard, near head of Alvin Canyon, R/ V Atlantis Cruise 283, Sta. Sl-3, coll. H.L. Sanders, Chief Scientist, 28 Aug 1962, 39°58.4′N, 70°40.3′W, 300 m, holotype ( LACM-AHF Poly 0564), 21 paratypes ( LACM-AHF Poly 0565). GoogleMaps — Near Block Canyon, R / V Chain, Cruise , 58, coll. H.L. Sanders, Chief Scientist, Epibenthic sled, Sta. Ch 105-B, 05 May 1966, 39°56.6′N, 71°03.6′W, 530 m, (62, LACM-AHF Poly 12721). GoogleMaps — Off New England, U.S. North Atlantic ACSAR Program , coll. G.W. Hampson, Chief Scientist. Sta. 12: Cruise NA-1, Rep. 1, 15 Nov. 1984, 39°54.32′N, 70°55.09′W, 558 m (2, USNM 1660929 About USNM ) GoogleMaps ; Cruise NA-2, Rep. 1, 04 May 1985, 39°54.31′N, 70°55.04′W, 551 m (3, USNM 1660930 About USNM ) GoogleMaps ; Rep. 2, 04 May 1985, 39°54.26′N, 70°55.07′W, 555 m (1, USNM 1660931 About USNM ) GoogleMaps ; Cruise NA 4, Rep. 3, 30 Nov 1985, 39°54.32ʹN, 70°55.12ʹW, 544 m (2, juv., USNM 1660932 About USNM ) GoogleMaps ; Cruise NA-5, Rep. 2, 06 May 1986, 39°54.27′N, 70°55.17′W, 548 m (2. USNM 1660935 About USNM ) GoogleMaps ; Cruise NA-6, Rep. 2, 30 Jul 1986, 39°54.26′N, 70°55.07′W, 559 m (1, USNM 1660933 About USNM ) GoogleMaps ; Rep. 3, 30 Jul 1986, 39°54.24ʹN, 70°55.09ʹW, 563 m (1, juv., USNM 1660934 About USNM ) GoogleMaps .
Comments on the material examined. Evaluation of the materials here referred to Chaetocirratulus gayheadius has proven to be difficult and complex. The type collection from R/V Atlantis Sta. Sl-3 at a depth of 300 m off the northeastern US reported by Hartman (1965) consists of the holotype and 21 paratypes. Of these, the holotype is regenerating the pre-setiger region and likely several anterior setigers and therefore cannot be used to characterize the species. The paratypes include juveniles of various sizes as well as small anterior and posterior fragments. None of the paratypes contain more than 30 setigers and none are sexually mature. As such, apart from characterizing post larvae and juveniles of the species, the type collection cannot by itself be used to characterize C. gayheadius . In contrast, the nearby sample from R/V Chain Sta. Ch 105-B from a depth of 530 m reported as Chaetozone gayheadia by Hartman & Fauchald (1971) includes a full range of sizes, the smallest of which overlap the juvenile paratypes from the type collection in size and shape. In addition, larger specimens with up to 51 setigers are available to characterize the adult morphology of the species and allow it to be compared with its congeners. Both collections are from adjacent upper continental slope depths and are in proximity to submarine canyons. A few specimens from the New England upper continental slope collected as part of the ACSAR program are also available and agree with the morphology of the larger specimens from Sta. Ch 105-B. In order to characterize the species, an illustrated size range of the types and additional specimens is provided. Descriptions are provided for both the type specimens and the materials from Sta. Ch 105-B. An additional sample reported by Hartman & Fauchald (1971) as Chaetozone gayheadia from nearby Veatch Canyon, from R/V Chain Sta. Ch-87 at a depth of 1102 m represents a different species (see below). Chaetocirratulus gayheadius thus appears to be limited to upper slope depths of about 300– 600 m.
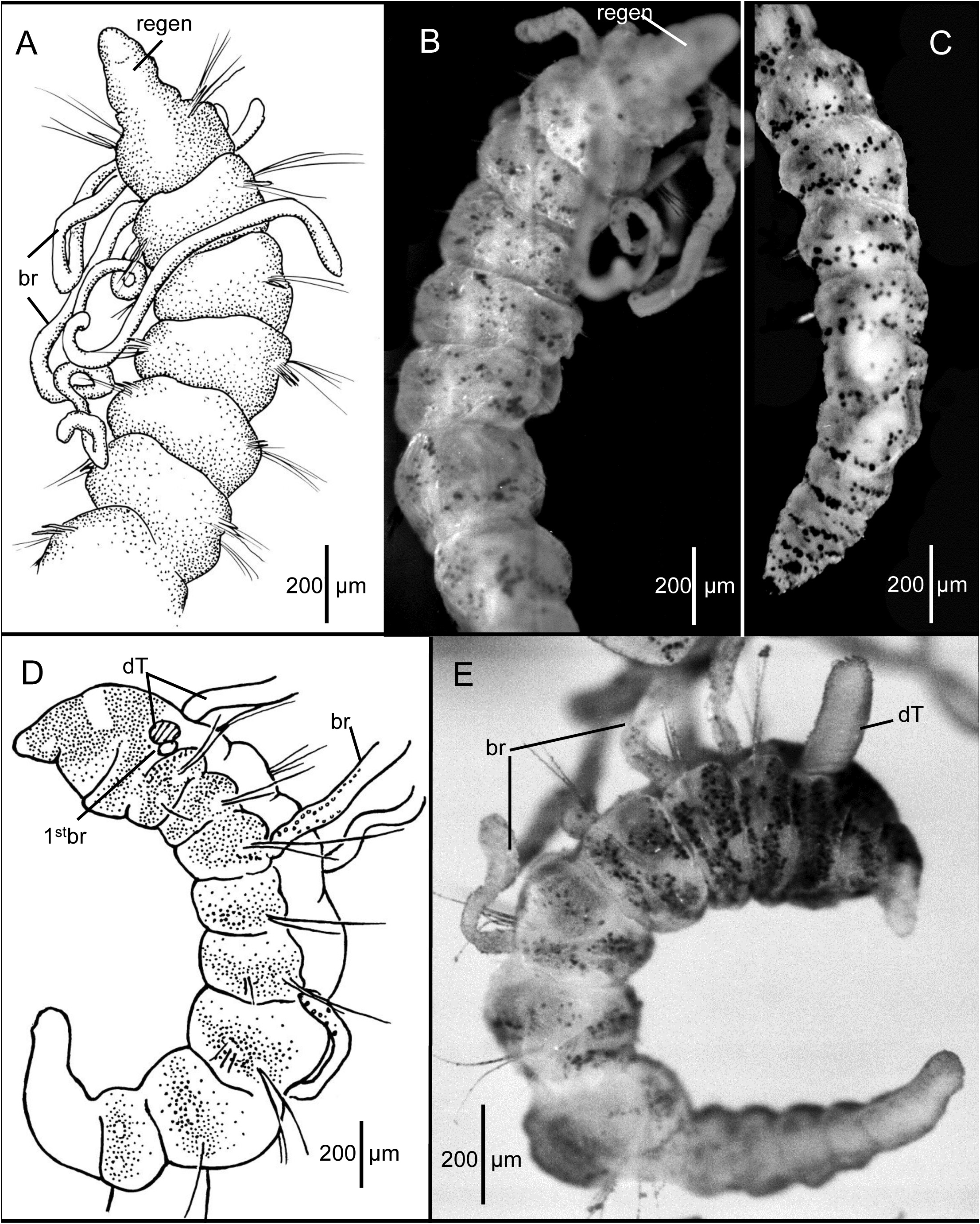
Description of the holotype (LACM-AHF Poly 0564). Holotype regenerating pre-setiger region ( Fig. 3A–B View FIGURE 3 ), complete, with 30 setigers, 5.6 mm long and 0.37 mm wide across anterior segments. Body slender throughout; anterior segments not expanded, individual segments distinctly separated from one another along most of body ( Fig. 3A–C View FIGURE 3 ) but not moniliform, becoming crowded and narrower in last few segments ( Fig. 3C View FIGURE 3 ). No dorsal or ventral grooves or depressions. Cross section of most segments rounded and slightly flattened dorsoventrally. Parapodia lateral throughout, with rami clearly separated. Color in alcohol: opaque white, without any pigmentation.
Prostomium and peristomium regenerating ( Fig. 3A–B View FIGURE 3 ), merged together, narrow, not differentiated; mouth a narrow slit. Dorsal tentacles not regenerated. Branchiae arise just posterior to and dorsal to notosetae on right setigers 1–3 and on left setigers 1 and 3 ( Fig. 3A–B View FIGURE 3 ). No branchiae more posteriorly, except on setiger 5.
Setae include smooth capillaries and sharp-tipped acicular spines. Notosetae of setigers 1–5 all capillaries, 3–4 per fascicle, each about as long as diameter of body, rather stiff. From setiger 6 notopodia provided with 1–2 pale, slender, curved acicular spines and 1–3 stiff capillaries. Notosetae of last 4–5 setigers long, dorsally oriented stiff capillaries. Neurosetae of setigers 1 to last (30) 2–3 pale, weakly curved acicular spines and 1–2 stiff capillaries. Neurosetae shorter than notosetae throughout.
Posterior segments tapering to pygidium ( Fig. 3C View FIGURE 3 ). Pygidium a small, rounded ventral lobe enclosing terminal anus.
Methyl green staining. Body stains turquoise blue in irregular pre-setiger and postsetal areas; speckled bands present, mostly concentrated on anterior segments ( Fig. 3B View FIGURE 3 ). Posterior setigers and pygidium with denser concentration of speckles ( Fig. 3C View FIGURE 3 ). Branchiae irregularly speckled. Regenerating anterior end pale, not staining.
Remarks on the holotype. The holotype is regenerating the pre-setiger region and an undetermined number of anterior setigers. Since none of the paratypes from R/V Atlantis Sta. Sl-3 have neuropodial acicular spines before setiger 5, it is unlikely that setiger 1 of the regenerating holotype is the actual first setiger. Therefore, it is likely that in addition to the pre-setiger region, the holotype is also regenerating at least four anterior setigers. For this reason alone, the holotype cannot be used to characterize the species.
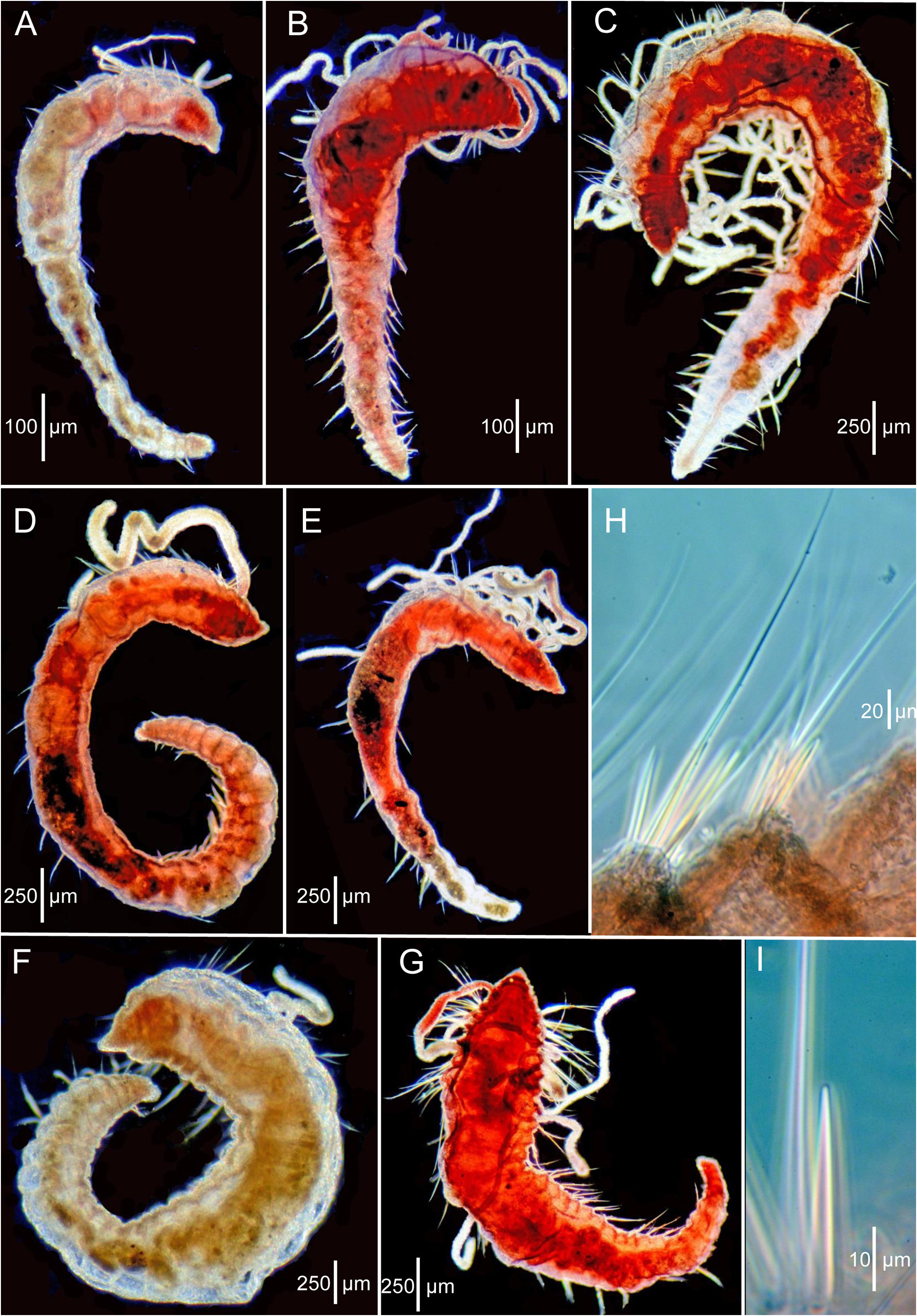
Description of the paratypes (LACM-AHF Poly 0565). All paratypes generally well preserved and in good condition. Most setae reasonably intact, many specimens with branchiae and a few with dorsal tentacles. Of 21 paratypes, six complete, nine anterior or posterior fragments, and six short complete, with enlarged anterior ends and narrow posterior ends ( Figs. 3D–E View FIGURE 3 , 4A–C View FIGURE 4 ).
Complete specimens range from 1.6 to 5.0 mm long and with 7 to 26 setigers. Greatest width of any paratype ca. 0.5 mm, not 1 mm as stated by Hartman (1965). Widest part of body first 1–7 anterior segments, then tapering posteriorly ( Figs. 3D–E View FIGURE 3 ; 4A–D View FIGURE 4 ). Color in alcohol: pale to whitish; brown branchiae mentioned by Hartman (1965) not apparent, but may have faded or been lost.
Body shape generally linear, with 2–7 anterior segments largest, sometimes inflated depending on specimen; middle and posterior segments narrower, generally tapering posteriorly ( Figs. 3D–E View FIGURE 3 ; 4A–D View FIGURE 4 ); anterior and some middle segments generally rounded or weakly moniliform, wider than long; most middle and posterior segments of larger specimens narrow, crowded, and tapering evenly towards simple narrow pygidium, rounded posteriorly ( Fig. 4D View FIGURE 4 ).
Prostomium conical, tapering to narrow bluntly rounded apex ( Figs. 3D–E View FIGURE 3 ; 4A–F View FIGURE 4 ); eyespots absent; nuchal organs curved slits on posterior lateral margin. Peristomium dorsally elevated, producing low dorsal crest; broader than prostomium; with two rings denoted only by lateral grooves in paratypes. Dorsal tentacles and first pair of branchiae arising from posterior margin of second peristomial ring; first branchiae located lateral to dorsal tentacles ( Figs. 3D–E View FIGURE 3 ; 4E View FIGURE 4 ). Subsequent branchiae arising dorsal to and posterior to notosetae on following setigers.
Parapodia lateral throughout, rami well separated with setae emerging directly from body wall but anterior setigers with a bulged or swollen area providing a shoulder effect in some specimens. Anterior setae all long, simple capillaries, ca. 5–10 per noto- and neuropodia. Acicular spines typically present in neuropodia from setigers 5–10 and notopodia from setigers 7–11. Small post-larval specimens with either no acicular spines or from setiger 7–8 in neuropodia and none in notopodia. Spines one per podia initially, increasing to 2 or 3 in posterior segments; accompanied by 2 or 3 capillaries. Individual spines relatively straight, tapering to pointed tip ( Fig. 4G View FIGURE 4 ).
Six short anterior ends with 6–7 large, rounded segments followed by narrow trunk with 2–7 narrow, crowded segments ( Figs. 3D–E View FIGURE 3 ; 4A–C View FIGURE 4 ). These paratypes identified as regenerates and as evidence of asexual reproduction by Petersen (1999). However, these paratypes more likely represent post-larval growth patterns based on similar and more advanced specimens from Sta. Ch 105-B (see below).
Methyl green staining. All specimens stain, some more brightly than others. Dorsal tentacles usually stain along outer edges of ciliated groove, visible as two fine lines, turquoise to dark blue. Branchiae stain dark turquoise to dark blue, some but not all with small discrete speckles along one side, rather than evenly distributed ( Fig. 3D–E View FIGURE 3 ). Pre-setiger region with a general mixture of dark blue and turquoise blue; coarser dark blue dots on a background of finer turquoise dots, with front of domed part of prostomium around the nuchal organs and extreme tip of the prostomium unstained or at most only lightly stained ( Fig. 3D View FIGURE 3 ). Individual segments stain anterior to the parapodia and in an irregular transverse postsetal ring laterally and ventrally ( Fig. 3D–E View FIGURE 3 ). Presetal rings present on some, but less dense. Areas where setae emerge unstained.
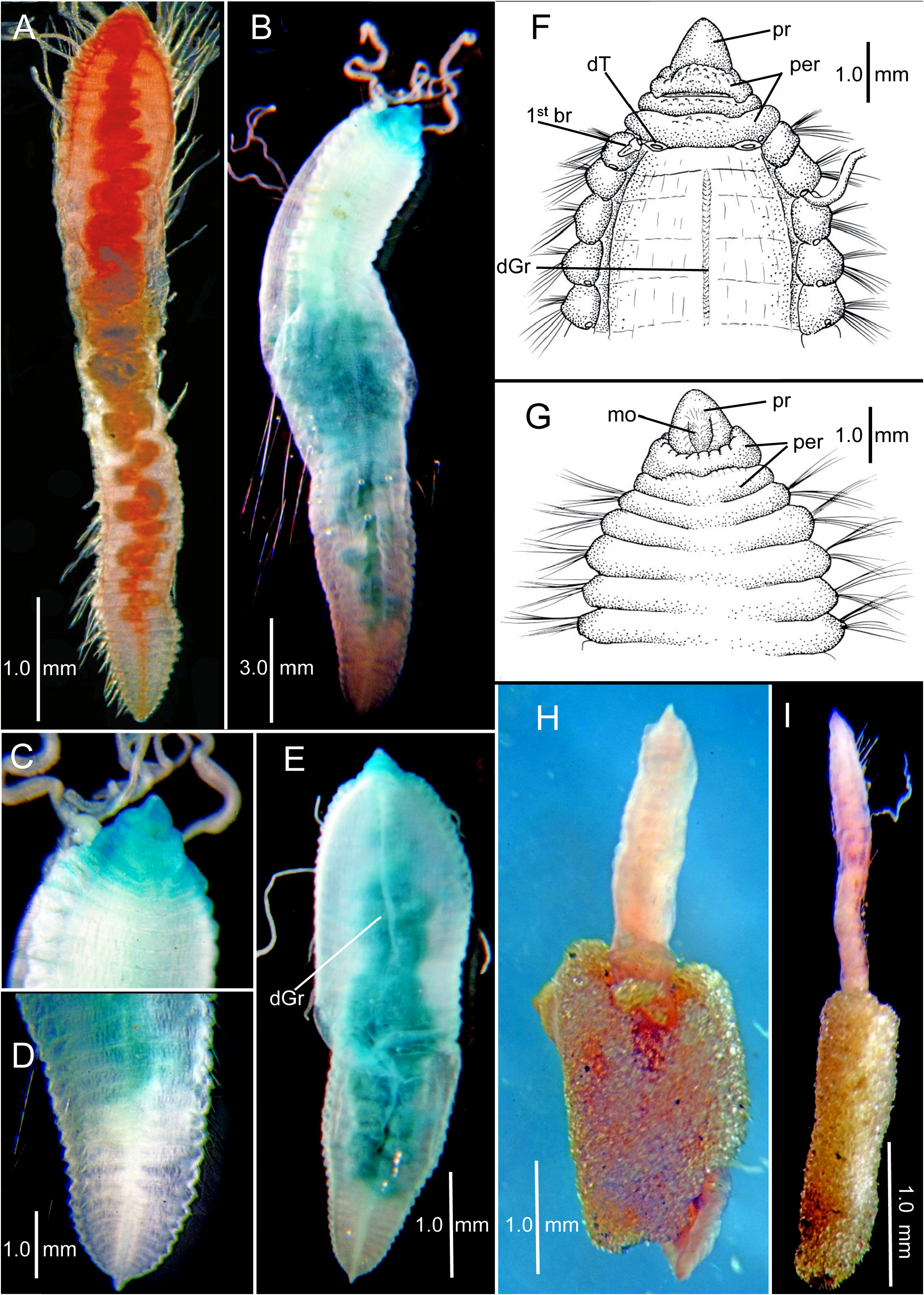
Description of non-type specimens from R/V Chain Sta. Ch 105-B (LACM-AHF Poly 12722). A wide range of sizes and shapes available ( Figs. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 ). Smaller specimens closely resemble small post-larval paratypes in having a thickened pre-setiger region and a few enlarged anterior setigers followed by a narrowing set of middle and posterior setigers ( Fig. 5A–B View FIGURE 5 ); with growth, the middle and posterior segments become thicker, resulting in a more consistent width along body ( Fig. 5C–F View FIGURE 5 ). Specimens illustrated in Figure 5 View FIGURE 5 measure 2.5–4.9 mm long with 19–34 setigers ( Table 1 View TABLE 1 ). A second slightly larger set of specimens illustrated in Figure 6A–F View FIGURE 6 with thickened part of body extending through anterior and middle segments and a narrow, dorsoventrally flattened posterior region. These specimens 3.1–5.2 mm long with 30–42 setigers ( Table 1 View TABLE 1 ); largest specimens illustrated in Fig. 7A–F View FIGURE 7 . Specimen in Fig. 7A View FIGURE 7 , 6.0 mm long with 40 setigers; largest specimen in sample 10.25 mm long with 51 setigers ( Fig. 7B–D View FIGURE 7 ); specimen in Fig. 5E View FIGURE 5 , 7.3 View FIGURE 7 mm long with 46 setigers; specimen in Fig. 7F–G View FIGURE 7 7.0 mm long with 40 setigers. Three complete specimens partially contained within tubes consisting entirely of small sand particles ( Fig. 7H–I View FIGURE 7 ). Color in alcohol white.
With growth, rounded anterior setigers characteristic of post-larvae and juveniles become shorter and wider; middle segments become larger, then narrow again posteriorly with posterior segments becoming wide and dorsoventrally flattened. Largest specimens assume a thickened sausage shape with most segments short and crowded along entire body ( Fig. 7A–E View FIGURE 7 ). Largest specimens with anterior dorsal segmental surface relatively smooth with indistinct segmental grooves; in addition a narrow mid-dorsal groove develops along anterior and middle segments ( Fig. 7E–F View FIGURE 7 ) and a mid-ventral ridge line develops along middle and posterior segments.
Pre-setiger region of adults relatively short, wider than long; about as long as first four setigers ( Fig. 7C, E–G View FIGURE 7 ). Prostomium broadly triangular, narrowing to rounded tip ( Fig. 7F–G View FIGURE 7 ); eyespots absent; nuchal organs posterior lateral slits, not pigmented. Peristomium with three rings observed dorsally ( Fig. 7F View FIGURE 7 ) and two rings ventrally ( Fig. 7G View FIGURE 7 ). Dorsally, posterior ring and setiger 1 clearly separated by an intersegmental groove ( Fig. 7F View FIGURE 7 ); ventrally, separation of anterior ring apparent laterally and dorsally, but merged with second ring ventrally forming posterior lip of mouth ( Fig. 7G View FIGURE 7 ); several specimens with dorsal separation between first and second rings weak or indistinct; dorsal tentacles arising from posterior margin of third peristomial ring ( Fig. 7F View FIGURE 7 ); first pair of branchiae lateral to dorsal tentacles. Second pair of branchiae arising dorsal to and posterior to notosetae on setiger 1 ( Fig. 7F View FIGURE 7 ); subsequent branchiae arising in similar location on following setigers.
Parapodia swollen lateral lobes with noto- and neuropodia distinctly separated; pre- and postsetal lobes absent. Setae include capillaries and acicular spines; some specimens with a few long, natatory-like capillaries ( Fig. 7B View FIGURE 7 ). Acicular spines all straight, tapering to narrow tip ( Figs. 4G View FIGURE 4 , 5H–I View FIGURE 5 ); neuropodial spines always occur more anteriorly than notopodial spines ( Table 1 View TABLE 1 ). Except for largest specimens, neuropodial spines first present from setiger in first third of body, whereas notopodial spines arise more posteriorly in middle body segments. In largest specimens, origin of spines shifted more posteriorly to posterior third or fourth of body ( Table 1 View TABLE 1 ). Smallest specimens with up to three neuropodial spines in a fascicle, whereas largest specimens, with only a single spine present, sometimes missing or skipping segments. For example, specimen illustrated in Fig. 5F–G View FIGURE 5 with 40 setigerous segments with a single neuropodial spine first present from setiger 21 with spines missing from two subsequent segments, then resuming. These observations suggest inconsistent spine development with segmental growth in larger specimens. Setae of larger specimens include 7–8 long capillaries in anterior notopodia and 3–4 in posterior notopodia in addition to a single acicular spine; anterior neuropodia include 6–8 capillaries and posterior neuropodia with four capillaries plus an acicular spine in. Larger specimens have 1–2 additional long, natatory-like capillaries in anterior and middle notopodia ( Fig. 7B View FIGURE 7 ).
Posterior end tapers to a narrow, triangular shaped pygidium terminating in a simple rounded lobe ventral to the anal opening ( Fig. 7D View FIGURE 7 ).
Methyl green staining. Methyl green is concentrated on the pre-setiger region and first 3–4 setigers ( Fig. 7B–C, E View FIGURE 7 ) with the strongest stain on the ventral side of the prostomium and lateral margins of the peristomium ( Fig. 7C View FIGURE 7 ); additional stain occasionally retained laterally in grooves between anterior parapodia.
Comparative Remarks. The collection from Sta. Ch 105-B includes specimens ranging in size from about 2.5 to over 10 mm long and having 19 to 51 setigerous segments. These specimens overlap with smaller specimens from the type collection from Sta. Sl-3 and demonstrate a growth sequence from short post-larval forms to fully developed adults.
A general pattern of morphological change from postlarvae and juveniles to the adult body form is apparent when the full complement of specimens from Stations Sl-1 (types) and Ch 105-B are compared. Initially the postlarvae and juveniles have 6–7 enlarged anterior moniliform setigers and a developing trunk region with narrow crowded segments ( Figs. 3D–E View FIGURE 3 , 4A–C View FIGURE 4 ). With growth, the larger rounded anterior setigers become less distinct and more crowded, with the moniliform shape of separate segments disappearing; the body tapers posteriorly along a narrowing trunk region ( Figs 4D View FIGURE 4 , 5A–E View FIGURE 5 ). Exceptions to this pattern include the regenerating holotype that has apparently lost the enlarged anterior setigers ( Fig. 3A–C View FIGURE 3 ) and one paratype ( Fig. 4E View FIGURE 4 ) where most of the segments, while not moniliform, are narrow and crowded only near the posterior end.
With continued growth, the body of these worms becomes thicker ( Fig. 6A–F View FIGURE 6 ) with little or no difference in size anywhere along the body; in addition, the posterior segments become dorsoventrally flattened. The largest specimens are sausage-shaped with no apparent difference in width anywhere along the body ( Fig. 7A–E View FIGURE 7 ) except for a short pre-setiger region that is noticeably narrower than the following inflated and enlarged anterior setigers. This short and narrow anterior end on an inflated sausage-shaped body provides the adults of this species with a characteristic appearance not encountered in other cirratulids. The dorsal surface is relatively smooth on anterior segments with indistinct segmental grooves and a narrow dorsal groove. Juveniles have two peristomial rings, whereas in the large adults, the anterior ring of juveniles is dorsally separated into two rings resulting in three rings, but the anterior two rings are merged ventrally forming the posterior lip of the mouth.
Four species of Chaetocirratulus have been identified from samples along the U.S. Atlantic continental slope. Of these, C. gayheadius , C. hessleri n. sp., and C. tomaculus n. sp. all have three peristomial rings, but differ in the nature and fate of these rings on the dorsal and ventral surfaces. Only C. hessleri n. sp. has a distinct dorsal crest that interrupts the three rings, whereas in C. gayheadius and C. tomaculus n. sp. the three peristomial rings are complete and not interrupted by a crest. Chaetocirratulus gayheadius differs from C. tomaculus n. sp. in having the anterior dorsal segmental surface relatively smooth with indistinct segmental grooves and a narrow dorsal groove. In contrast, the dorsal surface of C. tomaculus n. sp. exhibits a distinct segmental pattern on the dorsal surface with deep intersegmental grooves and no dorsal groove. The morphology of the four local species of Chaetocirratulus is compared in Table 2 View TABLE 2 .
Biology. The sandy tubes found on three specimens from Sta. Ch 105-B is unusual. For most benthic cirratulids, tubes, when reported, are lined with thickened mucous or fine silt particles. Some species of Kirkegaardia have been reported to project their branchiae into the overlying water through openings in mucoid tubes ( Blake & Magalhães 2019). Other species of Kirkegaardia form burrows within mud balls that serve as refuge or habitat ( Blake 2016; Blake & Magalhães 2019). The presence of tubes consisting of tightly adhering clean sand particles in C. gayheadius ( Fig. 7H–I View FIGURE 7 ) suggests a form of particle selection and cementing of these into tubes by the worms, the purpose of which is unknown.
Although long, natatory-like setae are present on the largest specimens, none were found with gametes.
Distribution. Off New England, 300– 558 m.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chaetocirratulus gayheadius ( Hartman, 1965 )
| Blake, James A. 2022 |
Chaetocirratulus gayheadius : Blake 2018: 121
| Blake, J. A. 2018: 121 |
Cirratulus gayheadius :
| Petersen, M. E. 1999: 112 |
| Petersen, M. E. 1991: 592 |
Chaetozone gayheadia
| Hartman, O. & Fauchald, K. 1971: 109 |
| Hartman, O. 1965: 166 |