Alijinocaris muricola, Williams, 1988
|
publication ID |
https://doi.org/10.1080/00222930400002499 |
|
persistent identifier |
https://treatment.plazi.org/id/050D1914-FFDC-FF93-FE60-FCF9FF384DE0 |
|
treatment provided by |
Carolina (2021-03-04 16:16:28, last updated by Plazi 2023-11-01 23:07:50) |
|
scientific name |
Alijinocaris muricola |
| status |
|
AlIJinocaris muricola Williams, 1988
( Figures 2 View Figure 2 , 3 View Figure 3 , 8–14 View Figure 8 View Figure 9 View Figure 10 View Figure 11 View Figure 12 View Figure 13 View Figure 14 , 29 View Figure 29 )
Alυinocaris muricola Williams 1988, p 268 , Figures 3 View Figure 3 , 4 View Figure 4 , 7 View Figure 7 [type locality: West Florida Escarpment, Western Atlantic, 26 ° 019N, 84 ° 54.619E, 3277 m]; Shank et al. 1999,
p 246 (Table 1), Figure 2 View Figure 2 ; Kikuchi and Hashimoto 2000, p 146, 148 (key). Alυinocaris cf. muricola: Olu et al. 1996, p 371 (Table 3).
Material examined
Gulf of Mexico. DS Alυin: dive 1754, West Florida Escarpment , 26 ° 019N, 84 ° 54.619W, 3277 m, 15.10.1986, one male CL 6.4 mm (holotype; USNM 234288 About USNM ); same data, one female CL 6.4 mm (allotype; USNM 234289 About USNM ); dive 3636, Florida Escarpment, 29.10.2000, two males CL 7.9, 8.3 mm, 12 females CL 5.3–14.0 mm, one juvenile CL 4.3 mm (Dr C. Van Dover’s collection) GoogleMaps .
South Barbados. Diapisub (DS Nautile), DS 04: site Orénoque A, 10 ° 19.649N, 58 ° 53.339W, 1697 m, 27 December 1992, one female CL 11.8 mm (MNHN-Na 15052) GoogleMaps .
Gulf of Guinea. Zaïrov ( ROV Victor): dive 74-14, Régab site, west equatorial African margin, 05 ° 47.809S, 09 ° 42.609E, 3151 m, 27–28 December 2000, claw jaw, one female CL 16.8 mm (MNHN-Na 14277); same data, one female CL 18.5 mm ( CBM-ZC 7042 ) GoogleMaps .
Biozaïre 1 ( ROV Victor): dive 81-5, Régab site, 10 January 2001, slurp gun 1, one male CL 5.7 mm, two females CL 6.8, 13.2 mm, four juveniles CL 3.7–5.3 mm (MNHN-Na 14278).
Biozaïre 2 ( ROV Victor): dive 146-9, Régab site, 28 November 2001, slurp gun 1, three females CL 16.5–21.0 mm (including one ovigerous female CL 21.0 mm) (Ifremer); same dive, slurp gun 2-1, seven males CL 7.4–11.0 mm, 14 females CL 5.8–23.0 mm (including two ovigerous females CL 14.5, 21.5 mm), nine juveniles CL 2.9–4.7 mm (Ifremer); same dive, slurp gun 2-2, seven males CL 6.0– 9.2 mm, 18 females CL 7.8–23.0 mm, 12 juveniles CL 3.6–6.0 mm (Ifremer); dive 146–9, slurp gun 3, 20 males CL 5.3–15.7 mm, one juvenile CL 3.6 mm [Ifremer; one male and two females transferred to NHM (registration number 2004: 231–233); one male and three females to NSMT (registration number Cr 15776–15779); one male and two females to USNM (registration number 1020566); and one male and three females to ZMMU (registration number Ma 3303)]; dive PL 147-10 , Régab site, 1 December 2001, slurp gun 1–1, one male CL 16.7 mm; slurp gun 1–2, two females CL 21.3, 21.4 mm (Ifremer); slurp gun 3, two males CL 7.6, 8.6 mm, 18 females CL 7.2–21.1 mm, three juveniles CL 4.2–5.0 mm (Ifremer); same dive, slurp gun 5-1, four males CL 7.1–8.8 mm, 10 females CL 7.2–20.4 mm, two juveniles CL 5.1, 5.2 mm (Ifremer); slurp gun 5-2, four males CL 7.1–11.8 mm, one female CL 20.5 mm (Ifremer) .
Bioz-Recup ( RV Suroit): 2 km of Régab site, 05 ° 47.169S, 09 ° 41.999E, 3155 m, January 2003, MAC (‘‘module autonome de colonisation’’) 10–147, three juveniles CL 3.8–4.8 mm (MNHN-Na 150539; MAC 10–151, two juveniles CL 3.8, 4.0 mm (MNHN- Na 15054); MAC 10–159, two juveniles CL 3.8, 4.4 mm (MNHN-Na 15055). GoogleMaps
M 56 Cruise ( RV Meteor): stn GeoB 8203-1, TV-grab, Congo Fan, 04 ° 48.579S, 09 ° 54.519W, 3110 m, 10 December 2002, four females CL 9.6–23.7 mm ( SMF) GoogleMaps ; stn GeoB 8212-2, TGV, Congo Fan , 04 ° 48.569S, 09 ° 54.509W, 3113 m, 17 December 2002, one ovigerous female (CL 21.3 mm) ( SMF) GoogleMaps .
Description
Body moderately robust.
Rostrum ( Figures 8A View Figure 8 , 10A View Figure 10 , 11 View Figure 11 A–G) directed forward, weakly curved dorsally or straight, 0.40–0.80 of carapace length in males, 0.30–0.65 in females, usually reaching to second segment of antennular peduncle in females, occasionally overreaching distal end of antennular peduncle in males; dorsal margin armed with 10–17 teeth, including 6–10 teeth on rostrum proper and four to six moderately large teeth on carapace posterior to orbital margin, posteriormost tooth arising from 0.34–0.40 of carapace length; ventral margin armed usually with 3–13 small teeth on anterior 0.30–0.70 (occasionally unarmed in large specimens with abnormally short rostrum). Carapace ( Figures 8A View Figure 8 , 9A View Figure 9 , 10A, C View Figure 10 ) 0.69–0.83 times as wide as long; postrostral median ridge moderately high, extending to 0.75–0.80 of carapace length, dorsal angle about 155 °; pterygostomian tooth strongly produced anteriorly in large specimens (CL. 13 mm), far beyond tip of antennal tooth ( Figures 8A View Figure 8 , 10A View Figure 10 , 11B, C, D, F View Figure 11 ); postantennal groove relatively deep, almost parallel to horizontal plane of carapace; branchial region somewhat inflated, thus lateral face notably convex.
Third abdominal pleuron rounded ( Figure 10D View Figure 10 ) or occasionally with one to four tiny teeth posteroventrally ( Figures 8C View Figure 8 , 13A View Figure 13 ). Fourth abdominal pleuron ( Figures 8C View Figure 8 , 10D View Figure 10 , 13A View Figure 13 ) with one to four (most frequently two or three) posterolaterally. Fifth abdominal somite similarly armed with one strong posteroventral tooth and two to five additional smaller teeth. Sixth abdominal somite 1.50–1.70 times longer than height. Telson ( Figures 8D View Figure 8 , 10E View Figure 10 ) nearly reaching to slightly overreaching posterior margin of uropodal endopod, length about 2.90–3.20 times anterior width and 4.90–5.20 times posterior width; armed with six to eight dorsolateral spines; posterior margin ( Figures 8E View Figure 8 , 10F View Figure 10 ) always moderately convex, armed with two pairs of spines at lateral angles and 12–14 plumose setae all longer than mesial pair of lateral spines.
Antennular peduncle ( Figures 8B View Figure 8 , 10B View Figure 10 ) moderately stout, second segment 1.90–2.10 times longer than wide. Antennal scale ( Figures 2A View Figure 2 , 8F View Figure 8 ) 0.48–0.51 of carapace length, 1.90–2.10 times longer than wide; lateral margin straight, slightly diverging anteriorly with dorsal median ridge; distolateral tooth moderately broad, directed forward, falling short of broadly rounded distal margin of blade.
First pereopod strongly polymorphic as illustrated ( Figures 9B, C View Figure 9 , 13 View Figure 13 B–H); greatest height of palm at most 0.64 times length of chela; dactylus shorter than palm in adults. Third pereopod ( Figure 9D View Figure 9 ) moderately slender; dactylus ( Figure 9E View Figure 9 ) with accessory spinules notably increasing in size distally; carpus 0.60–0.65 times as long as propodus; merus about 6.4 times as long as greatest height.
Size
Largest male CL 16.7 mm; largest female CL 21.5 mm, ovigerous females CL 14.5– 21.5 mm. Maximal CL 23.6 mm, TL 83 mm.
Variation
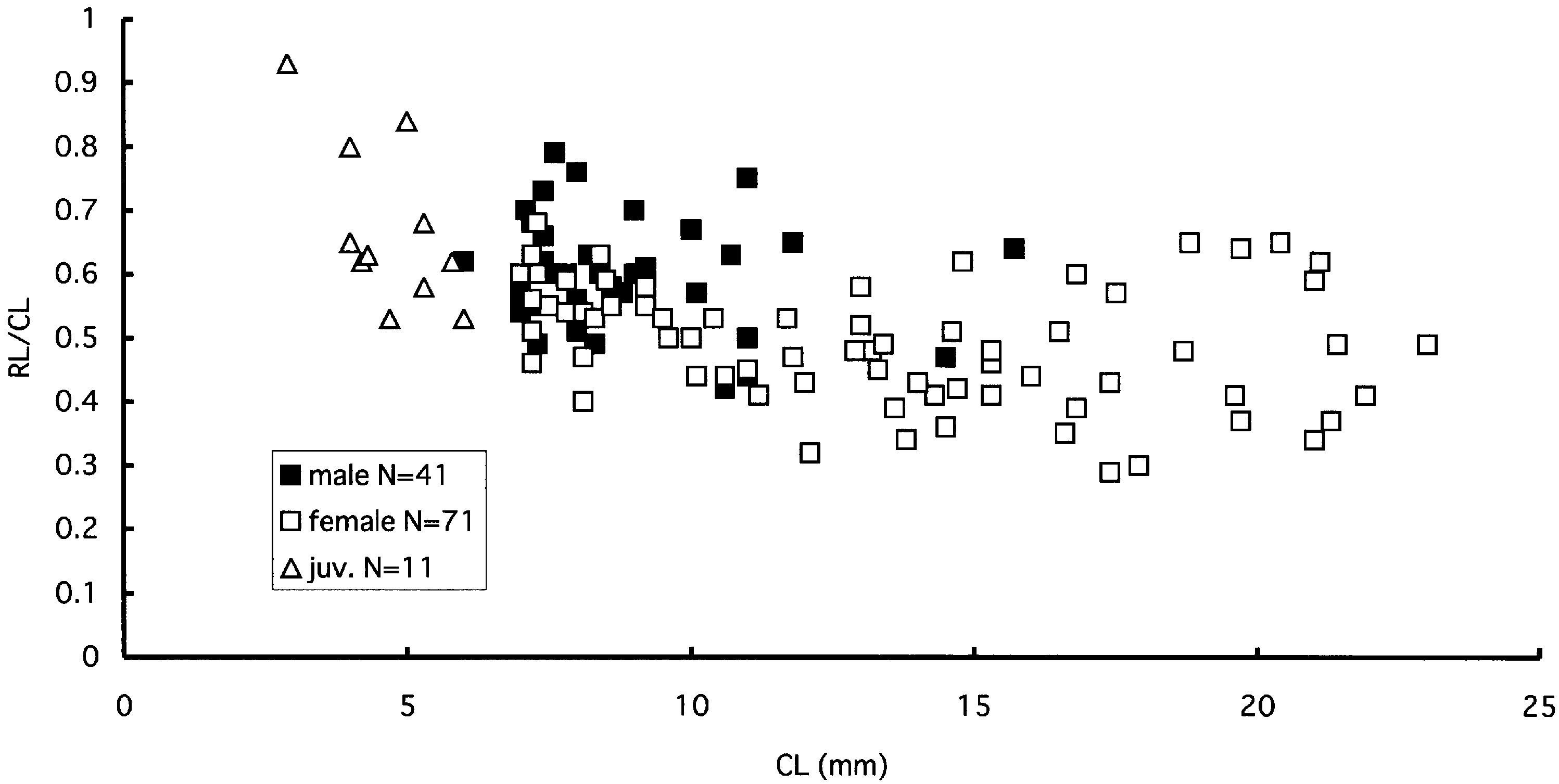
As is apparent from the above description, the length and armature of the rostrum vary considerably in this species (see Figures 11 View Figure 11 A–G, 12), but they are seemingly affected occasionally by injury and regeneration. The rostrum is sometimes more elongate in males than in females.
The third abdominal pleuron is variable from smooth to bearing at most four tiny denticles ( Figures 8C View Figure 8 , 10D View Figure 10 , 13A View Figure 13 ).
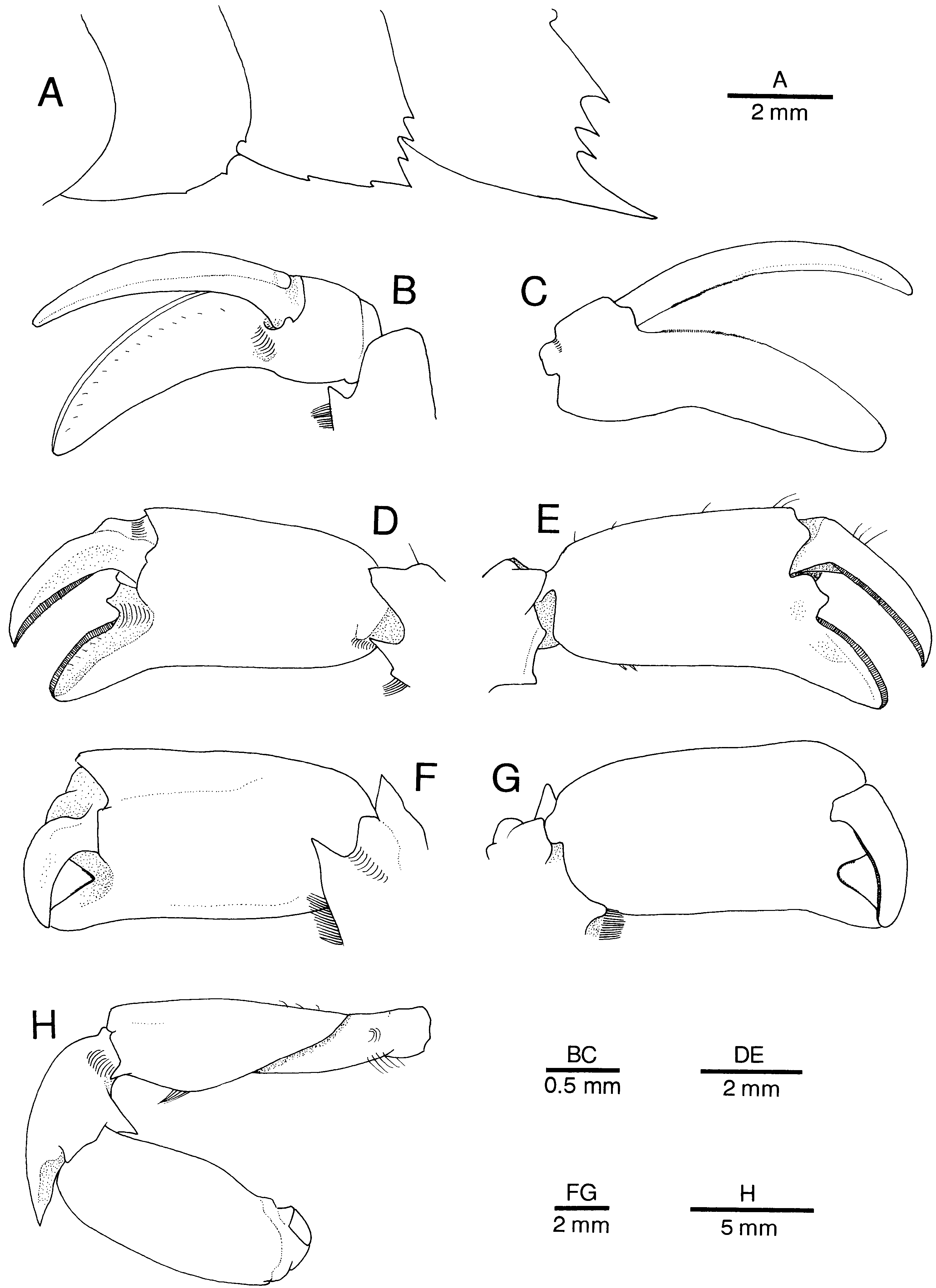
The first pereopod exhibits considerable polymorphism, not correlated to sex ( Figures 3C View Figure 3 , 9B, C View Figure 9 , 13 View Figure 13 B–H). A tendency for a decrease in the length of the fingers and an increase of the length and stoutness of the palm seem to be correlated to an increase in body size. The condition represented by Figure 13B and 13C View Figure 13 is limited to juvenile and immature specimens.
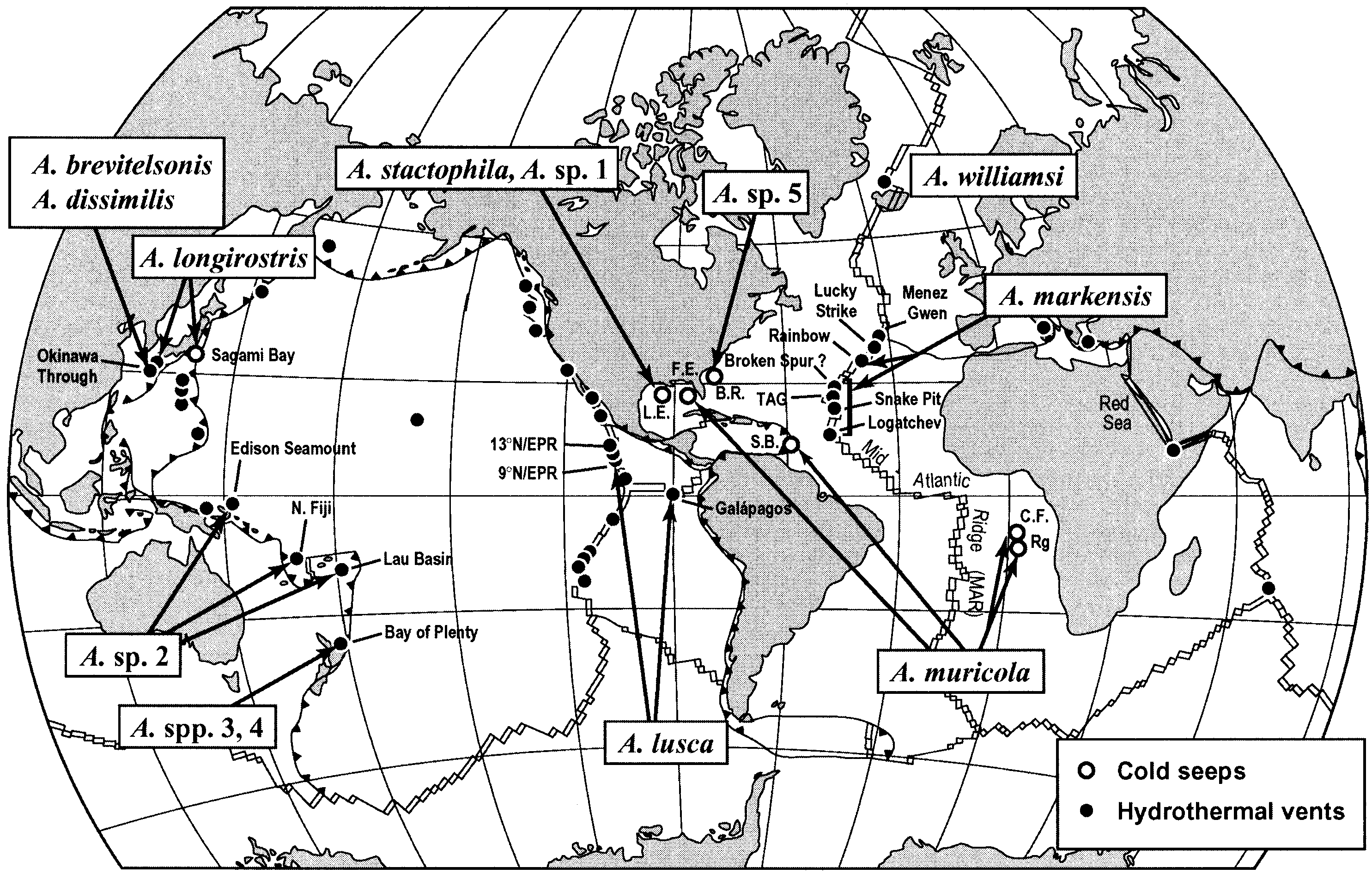
Distribution
Previously known with certainty only from the type locality, cold seeps of the West Florida Escarpment in the Gulf of Mexico , at a depth of 3277 m ( Figure 29 View Figure 29 ). The present material represents new records of this species from south Barbados (tropical western Atlantic ) and Régab (west equatorial African margin, 3113–3150 m). The specimen from Barbados significantly extends the bathymetric range to a shallower depth of 1697 m, and probably 1125 m (observation on video tapes) .
Ecology
The Régab site, near the Zaïre Channel, where A. muricola was newly discovered, is characterized by a community dominated by bivalves including two large mytilid Bathymodiolus spp. and vesicomyids ( R. von Cosel, unpublished data), and vestimentiferans Escarpia n. sp. (Andersen et al. forthcoming). The communities are distributed in aggregates over an area of ca 1 km 2. The substrata are mixed with soft reduced sediment and outcrops of carbonated concretions. The shrimps were over the mussel or the clam beds, or among the vestimentiferan Escarpia n. sp., or on the sediment ( Figures 14A, B View Figure 14 ). Other accompanying species are present: sea-anemones, galatheid Munidopsis spp., gastropod Phymorhynchus sp., chiridotid holothurians and zoarcid fish. The highest densities of the shrimp were recorded on the mussel beds (more than 300 individuals per m 2). The preliminary analysis of the stomach content of one specimen, using SEM, revealed the presence of fragments of diatoms and small foraminiferan drowned in dark mucus composed of very fine mineral particles. Four ovigerous females were collected; two carried 3774 and 1432 eggs, one was preserved intact and the fourth carried very few eggs. A small nematode, Chromadorita sp. ( Adenophorea), occurs among the eggs (A. Vanreusel, unpublished data).
The photographs taken at cold seeps on the Congo Fan area during the M 56 Cruise, show that the faunal community is generally similar to that of Régab site, except that the mytilid bivalves are apparently absent. The following are found: vesicomyid clam beds composed of relatively few living individuals, dense cluster of vestimentiferan (probably a species of Escarpia ), many galatheid lobsters, and chiridotid holothurians. Alυinocaris muricola lives overall among the Escarpia clusters, but also on the dark reduced sediment, occasionally covered with white bacteria mats.
The only specimen, collected from a subduction area with many mud volcanoes located on south Barbados accretionary prism (tropical western Atlantic), lives also on a bivalve community chiefly composed of two species of Bathymodiolus : B. boomerang Cosel and Olu, 1998 and B. sp. 1 (Cosel 2002) which are close to the species occurring on the African margin. In some active mud volcanoes, development of abundant communities composed of chemosynthetic species, vesicomyid bivalves and mytilid species Bathymodiolus boomerang and vestimentiferan worm Escarpia cf. laminata Jones, 1985 , which characterize an area of high methane discharge. Other bathyal and rather opportunistic species were present, such as the serpulid polychaete Neoυermilia sp., gastropods Phymorhynchus aff. alberti , geryonid crab Chaceon sp., lithodids Paralomis arethusa Macpherson, 1994 and Lithodes manningi Macpherson, 1994 , and galatheid crabs ( Olu et al. 1996; Warén and Bouchet 2001; Macpherson 1994). On the modiolid beds, Alυinocaris muricola were very abundant (more 300 individuals per m 2 on average), particularly on the Orénoque A site (1697 m). Analysis of the video tapes shows the occurrence of shrimps assignable to A. muricola on the El Pilar site (1125 m), which represents the shallowest bathymetric limit of this species at a depth of 1125 m. The deep mud volcano of Barbados, located some 400 km north from Barbados accretionary prism and at a depth of nearly 5000 m, was found to be colonized by bivalves Calyptogena sp., but no shrimp was observed there (K. Olu, personal communication).
Remarks
The holotype and allotype of A. muricola are young, the carapace length measuring 6.4 mm in length in both specimens. Specific features are not differentiated in the type specimens. In this study, the specific identity of A. muricola is established primarily by using topotypic adult specimens from the West Florida Escarpment, kindly provided by Dr C. Van Dover. Comparison of the topotypic specimens with the material from Barbados and the west equatorial African margin has revealed that there are no morphological differences among the three well-separated populations. Therefore, we refer the Barbados and western African populations to A. muricola .
Although Williams (1988) mentioned that the obscurely serrated pleural margin of the third abdominal somite is diagnostic for A. muricola , our examination has shown that the condition of the pleural margin is quite variable in this species. Even in the holotype, the left margin bears a minute denticle ventrally, while the right margin is smooth, without trace of teeth or denticles. In the allotype, the left margin bears three small teeth posteroventrally; the right margin also bears two small teeth posteroventrally. The occasional presence of minute denticles or teeth on the third abdominal pleuron is also known in A. markensis and A. longirostris (present study; Kikuchi and Ohta 1995). As mentioned before, A. muricola is closely allied to A. markensis , A. longirostris , and A. dissimilis sp. nov. Differences among the four species are discussed under the account of A. dissimilis sp. nov.
Alυinocaris sp. represented by the allotype of A. stactophila is also similar to A. muricola . Nevertheless, the absence of ventral teeth on the rostrum and stouter second segment of the antennular peduncle seem to distinguish Alυinocaris sp. from A. muricola .
Shank et al. (1999) presented an unconfirmed report of A. muricola at Logatchev on the Mid-Atlantic Ridge at a depth of 3010 m by citing a personal communication from A. Gebruk and A. Vereshchaka, while they mentioned also the occurrence of A. markensis at the same site. Later Gebruk et al. (2000a) considered the Alυinocaris shrimp found on the mussel beds on the Anya’s Garden site at Logatchev as an undescribed species. The authors related their species to A. muricola . However, the specific status of the Anya’s Garden population remains unclear, as no material from the site has been available for study.
Gebruk AV, Chevaldonne P, Shank TM, Lutz RA, Vrijenhoek RC. 2000 a. Deep-hydrothermal vent communities of Logatchev area (14 ° 459 N, Mid-Atlantic Ridge): diverse biotopes and high biomass. Journal of Marine Biological Association of the United Kingdom 80: 383 - 393.
Kikuchi T, Ohta S. 1995. Two caridean shrimps of the families Bresiliidae and Hippolytidae from a hydrothermal field on the Iheya Ridge, off the Ryukyu Islands, Japan. Journal of Crustacean Biology 15: 771 - 785.
Kikuchi T, Hashimoto J. 2000. Two new caridean shrimps of the family Alvinocarididae (Crustacea, Decapoda) from a hydrothermal field at the Minami-Ensei Knoll in the Mid-Okinawa Trough, Japan. Species Diversity 5: 135 - 148.
Macpherson E. 1994. Occurrence of two lithodid crabs (Crustacea: Decapoda: Lithodidae) in the cold seep zone of the south Barbados accretionary prism. Proceeding of the Biological Society of Washington 107: 465 - 468.
Olu K, Sibuet M, Harmegnies F, Foucher J-P, Fiala-Medioni A. 1996. Spatial distribution of diverse cold seep communities living on various diapiric structures of the southern Barbados prism. Progress in Oceanography 38: 347 - 376.
Shank TM, Black MB, Halanych KM, Lutz RA, Vrijenhoek RC. 1999. Miocene radiation of deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): evidence from mitochondrial cytochrome oxidase subunit I. Molecular Phylogenetics and Evolution 13: 244 - 254.
Waren A, Bouchet P. 2001. Gastropoda and Monoplacophora from hydrothermal vents and seeps; new taxa and records. The Veliger 44: 116 - 231.
Williams AB. 1988. New marine decapod crustaceans from waters influenced by hydrothermal, discharge, brine and hydrocarbon seepage. Fishery Bulletin 86: 263 - 287.
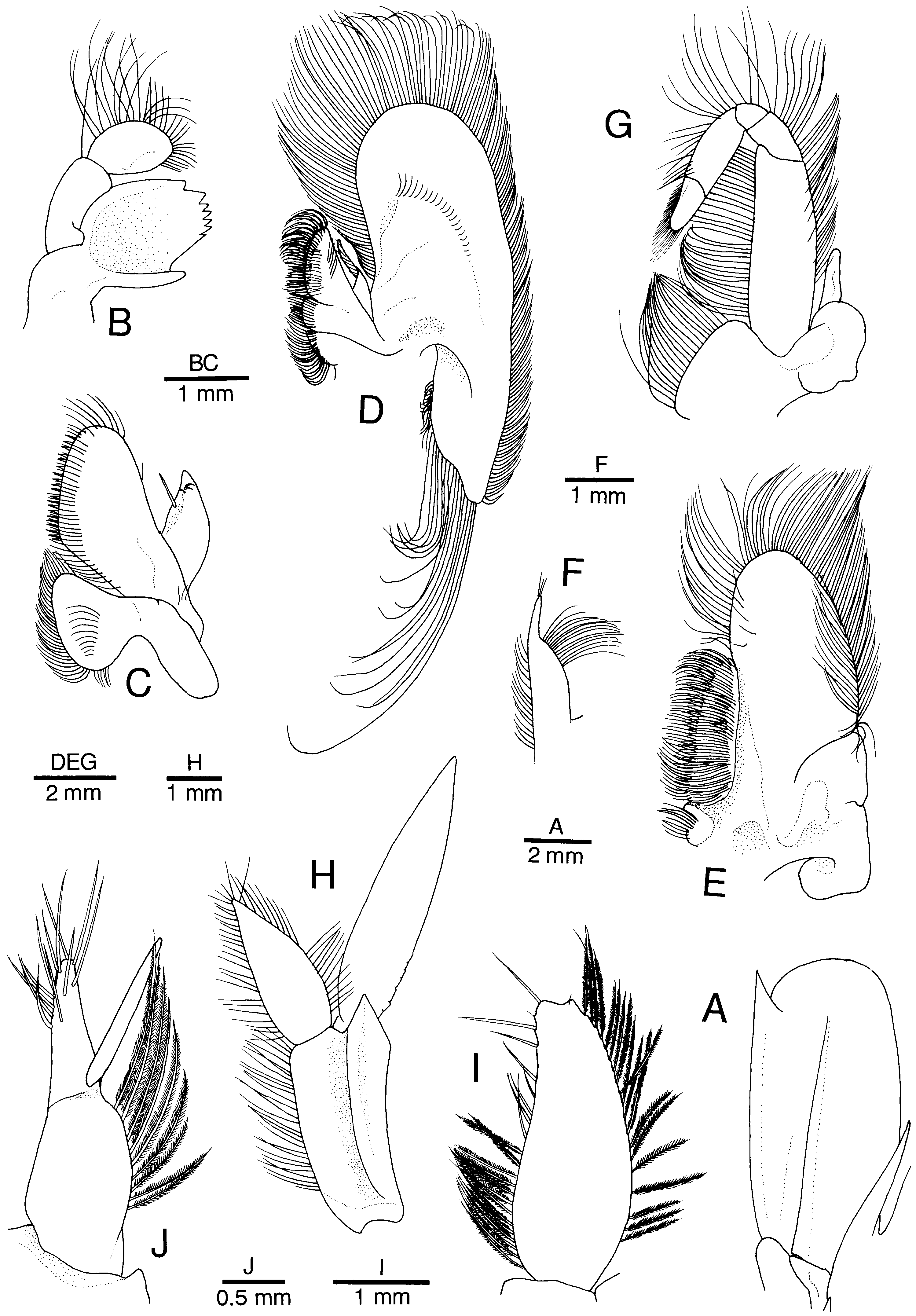
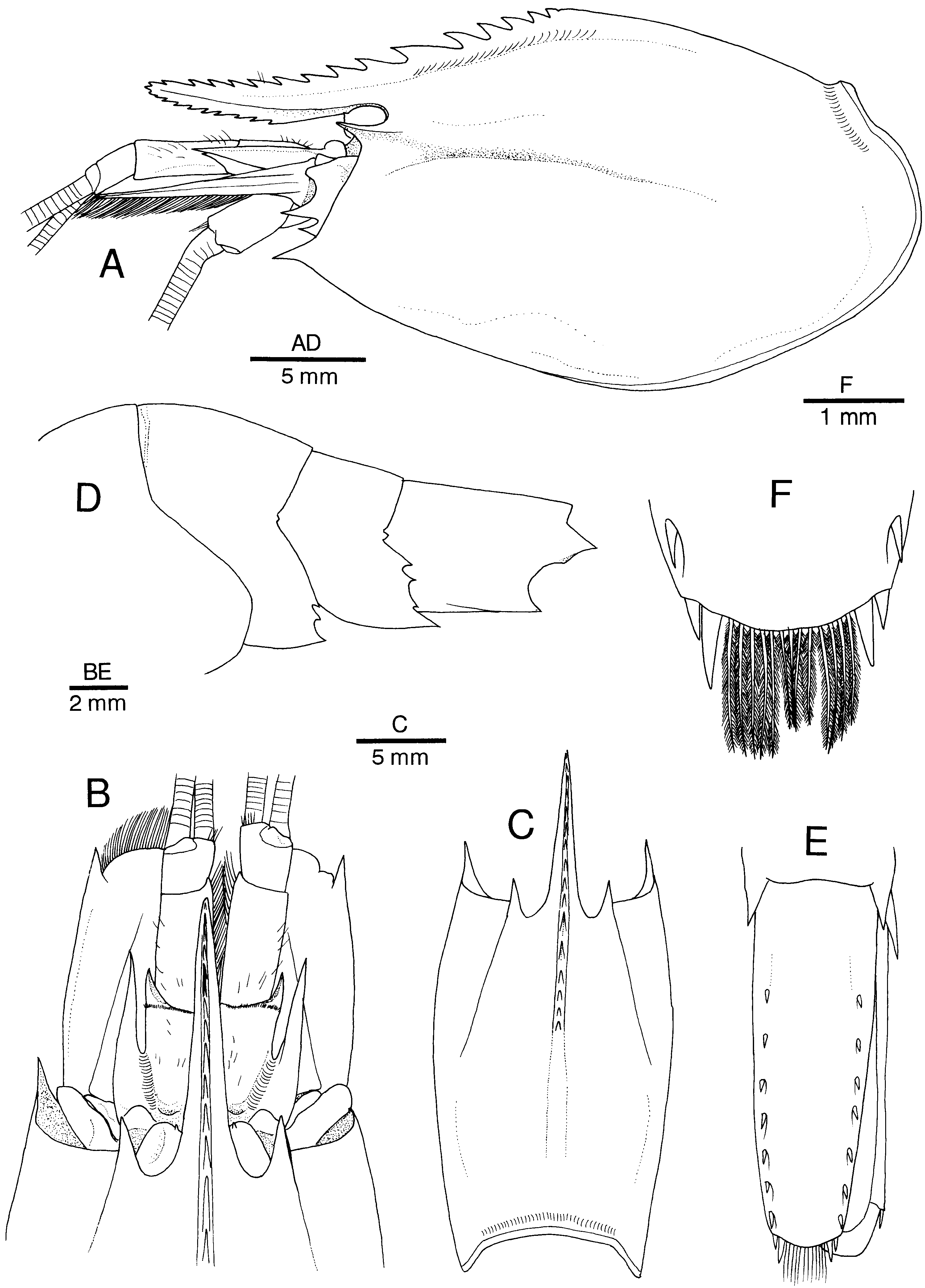
Figure 2. Alυinocaris muricola Williams, 1988. Left appendages. (A) Antennal scale, dorsal; (B) mandible, dorsal; (C) maxillule, ventral; (D) maxilla, ventral; (E) first maxilliped, ventral; (F) endopod of first maxilliped, dorsal; (G) second maxilliped, ventral; (H) first pleopod, ventrolateral; (I) endopod of first pleopod; (J) appendices interna and masculina of second pleopod, mesial. (A–H) Female from Régab site, west equatorial African margin (CL 16.8 mm; MNHN-Na 14277); (I, J) male from same locality (CL 15.4 mm; Ifremer).
Figure 3. Alυinocaris muricola Williams, 1988. Left thoracic appendages. (A) Third maxilliped, lateral; (B) coxa and antepenultimate segment of third maxilliped, dorsal; (C) first pereopod, lateral; (D) carpus of first pereopod, mesial; (E) second pereopod, lateral; (F) chela of second pereopod, dorsal; (G) third pereopod, lateral; (H) dactylus of third pereopod, lateral; (I) fourth pereopod, lateral; (J) fifth pereopod, lateral; (K) dactylus of fifth pereopod, lateral. Female from Régab site, west equatorial African margin (CL 16.8 mm; MNHN-Na 14277).
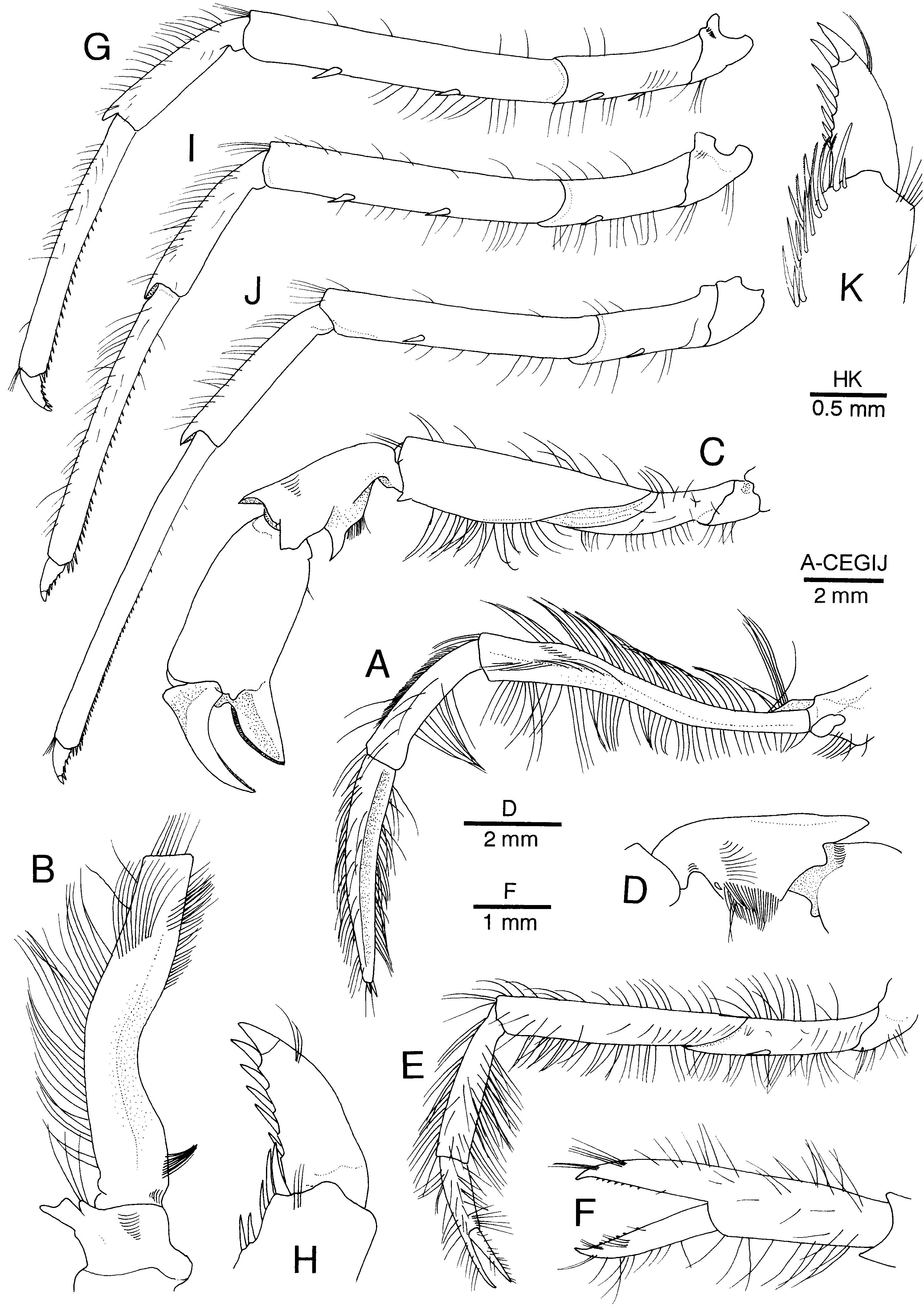
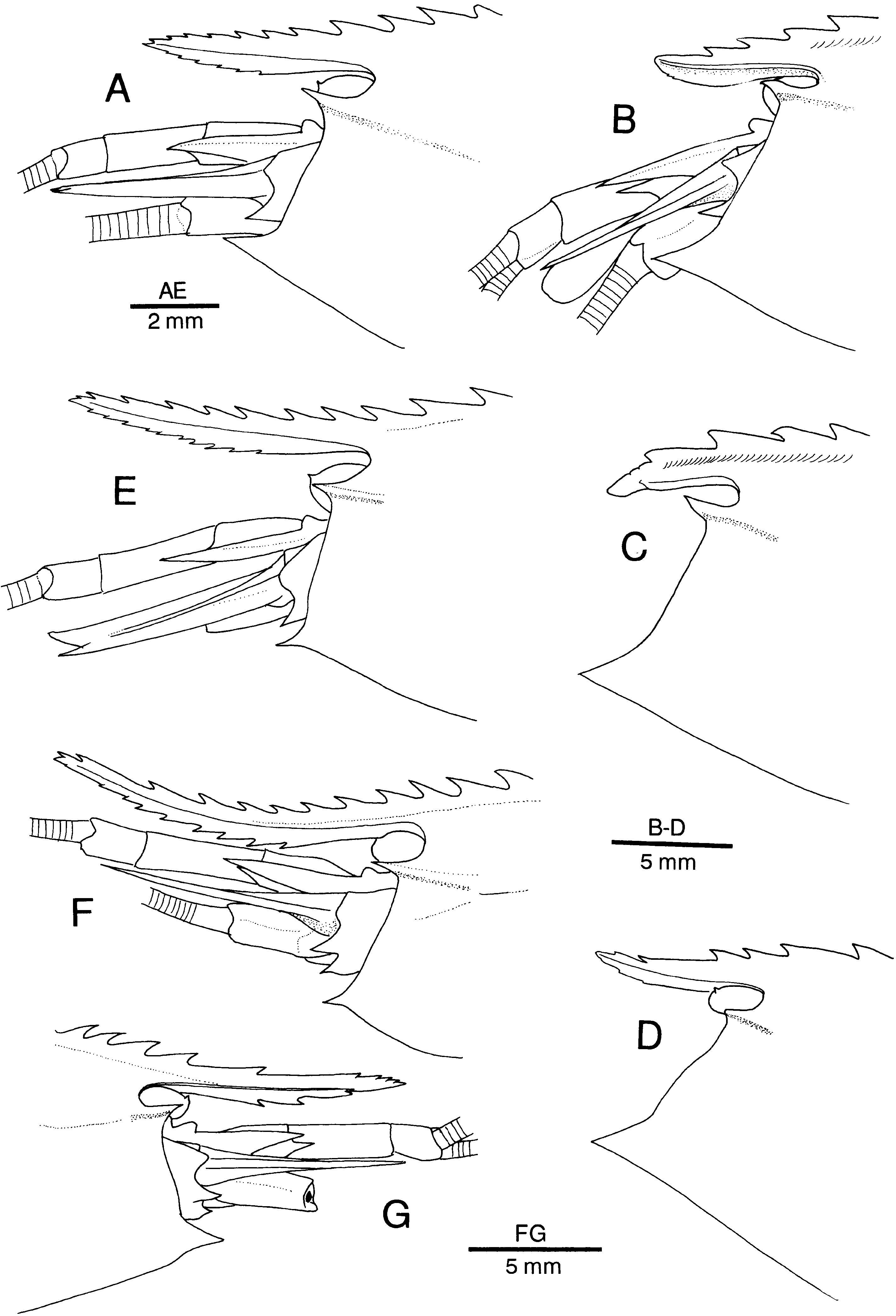
Figure 4. Alυinocaris lusca Williams and Chace, 1982. (A) Carapace and cephalic appendages, lateral (rostrum broken off); (B) anterior part of carapace and cephalic appendages, dorsal; (C) same, lateral; (D) carapace, dorsal (rostrum broken off); (E) third to sixth abdominal somites, lateral. (A, B, D, E) Paratype female from Rose Garden area, Galapagos Rift (CL 13.5 mm; USNM 184537); (C) paratype female (CL 8.6 mm; same lot).
Figure 7. Alυinocaris markensis Williams, 1988. (A) Left antennal scale, dorsal; (B) left first pereopod, lateral; (C) chela of left first pereopod, outer; (D) left third pereopod, lateral; (E) dactylus and distal part of propodus of left third pereopod, lateral. Female from site Les Ruches, Snake Pit, Mid-Atlantic Ridge (CL 16.7 mm; MNHN- Na 14282).
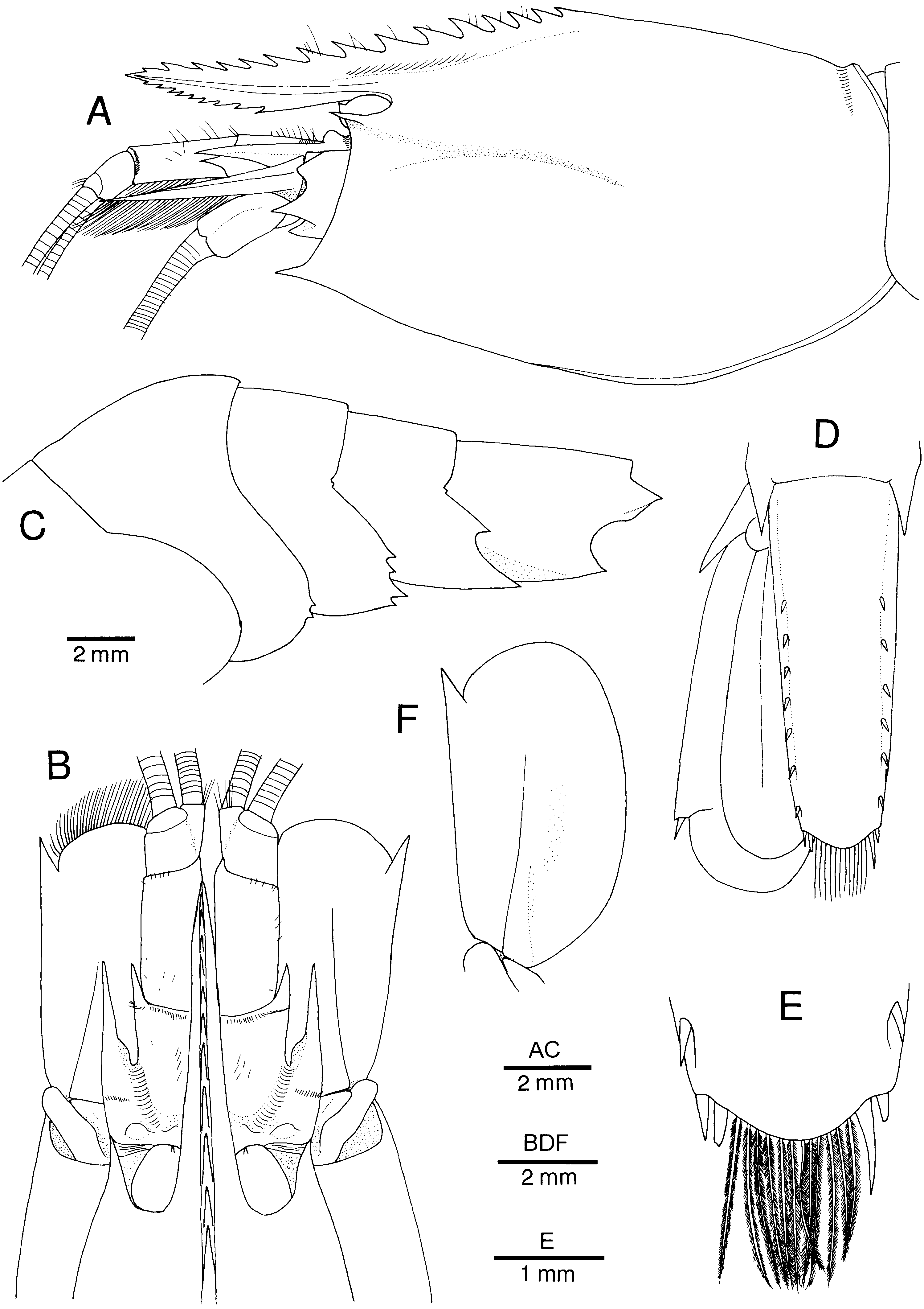
Figure 8. Alυinocaris muricola Williams, 1988. (A) Carapace and cephalic appendages, lateral; (B) anterior part of carapace and cephalic appendages, dorsal; (C) second to sixth abdominal somites, lateral (setae omitted); (D) telson and left uropod, dorsal (marginal setae on uropod omitted); (E) posterior part of telson, dorsal; (F) left antennal scale, dorsal (marginal setae omitted). Female from West Florida Escarpment (CL 13.8 mm; Dr C. Van Dover’s collection).
Figure 9. Alυinocaris muricola Williams, 1988. (A) Carapace, dorsal; (B) left first pereopod, lateral; (C) chela of left first pereopod, inner; (D) left third pereopod, lateral; (E) dactylus and distal part of propodus of left third pereopod, lateral. Female from West Florida Escarpment (CL 13.8 mm; Dr C. Van Dover’s collection).
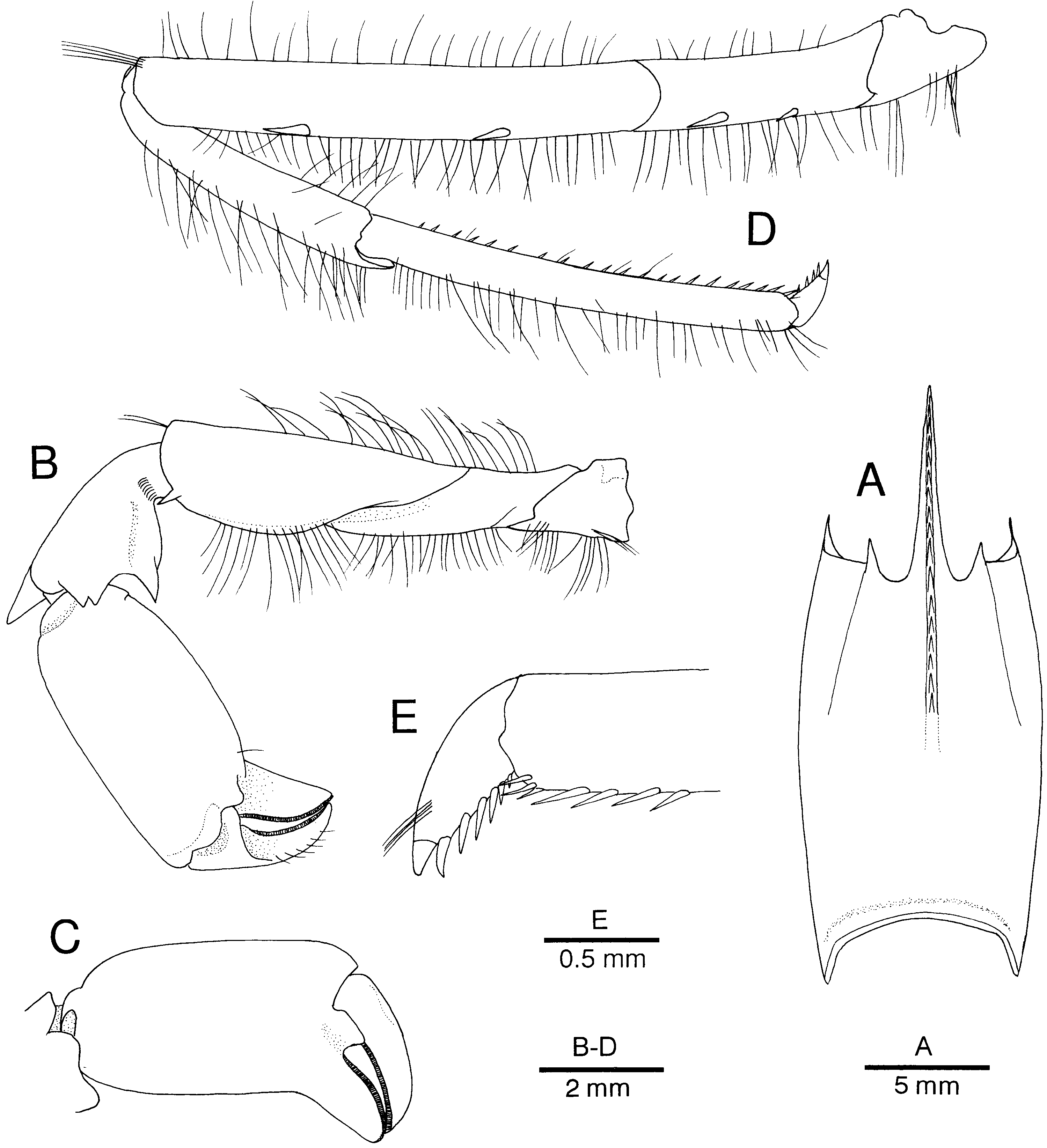
Figure 10. Alυinocaris muricola Williams, 1988. (A) Carapace and cephalic appendages, lateral; (B) anterior part of carapace and cephalic appendages, dorsal; (C) carapace, dorsal; (D) third to sixth abdominal somites, lateral; (E) telson and right uropod, dorsal; (F) posterior part of telson, dorsal. Female from Régab site, west equatorial African margin (CL 16.8 mm; MNHN-Na 14277).
Figure 11. Alυinocaris muricola Williams, 1988. Variation in development and armature of rostrum. Specimens from Régab site, west equatorial African margin (Biozaïre 2). (A) Female, dive 146-09, slurp gun 3 (CL 11.0 mm; Ifremer); (B) female from same dive, slurp gun 2 (CL 21.0 mm; Ifremer); (C) female from same lot (CL 22.0 mm); (D) female from dive 147-10, slurp gun 1 (CL 21.6 mm; Ifremer); (E) male, dive 146, slurp gun 3 (CL 10.0 mm; Ifremer); (F) male (CL 16.2 mm; Ifremer); (G) male, dive 146, slurp gun 3 (CL 15.3 mm; Ifremer).
Figure 12. Alυinocaris muricola Williams, 1988. Plot of proportional length of rostrum (RL/CL) against carapace length (CL).
Figure 13. Alυinocaris muricola Williams, 1988. (A) Third to fifth abdominal pleura, lateral (setae omitted); (B, D, F) chela of first pereopod, ventral (outer); (C, E, G) same, dorsal (inner); (H) entire first pereopod, lateral. Specimens from Régab site, west equatorial African margin. (A) Female from Zairov, dive 74-14 (CL 18.5 mm; CBM-ZC 7042); (B, C) female, Biozaïre 1, dive 81-5 (CL 13.2 mm; MNHN-Na 14278); (D, E) female from Zairov, dive 74-14 (CL 16.8 mm; MNHN-Na 14277); (F–H) female from Biozaïre 2, dive 146-09, slurp gun 2 (CL 21.0 mm; Ifremer).
Figure 14. (A) In situ photograph taken by the submersible Nautile, at a sulphide edifice of vent site Les Ruches, Snake Pit, Mid-Atlantic Ridge (3480 m) during Hydrosnake Cruise (PL 08): on the centre, one individual of Alυinocaris markensis Williams, 1988; other shrimps, Chorocaris chacei. Copyright Ifremer/Hydrosnake. (B) In situ photograph taken by the ROV Victor, at the cold seep site Régab, west equatorial African margin (3150 m) during Biozaïre 2 Cruise (PL 146): aggregation of Alυinocaris muricola Williams, 1988, among mytilid mussels Bathymodiolus sp. and tubeworm pogonophorans Escarpia n. sp.; lower left corner, coiled gastropods Proυanna sp. (fide A. Warén, Stockholm). Copyright Ifremer/Biozaïre 2. (C) Same data, Alυinocaris muricola Williams, 1988, among mussels Bathymodiolus sp. and actiniarians; lower right corner, limpet gastropods Paralepetopsis sp. (fide A. Warén). Copyright Ifremer/Biozaïre 2.
| ROV |
Museo Civico di Rovereto |
| NSMT |
National Science Museum (Natural History) |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| ZMMU |
Zoological Museum, Moscow Lomonosov State University |
| RV |
Collection of Leptospira Strains |
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Alijinocaris muricola
| Komai, Tomoyuki & Segonzac, Michel 2005 |
cf. muricola:
| Olu 1996: 371 |
muricola
| Williams 1988: 268 |