Abyssorchomene abyssorum ( Stebbing, 1888 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.825.1829 |
|
publication LSID |
lsid:zoobank.org:pub:2AA16E5D-428F-4827-9944-E2EDC3CF90FD |
|
DOI |
https://doi.org/10.5281/zenodo.14284130 |
|
persistent identifier |
https://treatment.plazi.org/id/05062C54-4065-9E25-0740-4CB5FCB2FEDB |
|
treatment provided by |
Felipe |
|
scientific name |
Abyssorchomene abyssorum ( Stebbing, 1888 ) |
| status |
|
Abyssorchomene abyssorum ( Stebbing, 1888) View in CoL View at ENA
Figs 2–9 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Orchomene abyssorum Stebbing, 1888: 676–679 , pl. 21.
Orchomenopsis abyssorum Sars 1895: 74 . — Stebbing 1906: 84, fig. 14. —? Chevreux 1900: 23–24. — Chevreux 1903: 92. —? Walker 1903: 224, 232. — Chevreux 1905: 7 (in list). —? Stephensen 1925: 125. — Chevreux 1935: 59–60 (in part, part = Abyssorchomene cf. abyssorum View in CoL ).
Anonyx abyssorum Della Valle 1893: 824 .
Orchomenella abyssorum Ruffo 1949: 10 (in list). – Schellenberg 1955: 192 (in list). — Barnard J.L. 1958: 96 (in list). —? Birstein & Vinogradov 1960: 188–189, 227, 232, fig. 8. — Gurjanova 1962: 433 (in key). — Hurley 1963: 125–126. — Barnard J.L. 1964: 86, 89 (in key). —? Birstein & Vinogradov 1964: 164. — Thurston & Allen 1969: 364. — Sanderson 1973: 37 (in list). —? Lowry & Stoddart 1994: 129, 180–181. — Vinogradov G. 1999: 1147, 1164–1165 (in part; part = Abyssorchomene scotianensis View in CoL ).
Orchomene abyssorum – Lowry & Bullock 1976: 94–95 (in part; part = Abyssorchomene scotianensis View in CoL ). — Shulenberger & Barnard 1976: 248. — Andres 1983: 209, 211–212. —? Austin 1985: 601 (in list).— Barnard & Karaman 1991: 508 (in part; part = Abyssorchomene scotianensis View in CoL ). —? Kaufmann 1992: 1–170. —? Bellan-Santini 1998: 145–146, 148.
Abyssorchomene abyssorum View in CoL – De Broyer 1983: 142–144 (in part; part = Abyssorchomene scotianensis View in CoL ). — Palerud & Vader 1991: 32 (in list). —? Jones et al. 1998: 1124–1125. —? Witte 1999: 143. —? Janssen et al. 2000: 3005, 3010–3011, 3013, 3015. — Thurston 2001: 358, 360, 362, 365, 369 (in part; part = Abyssorchomene scotianensis View in CoL ). —? Treude et al. 2002: 1284–1285, 1288. —? Horton 2005: 1. — Brandt et al. 2012: 144, 146, 152, 155 (biogeography). (in part). — Lowry & Kilgallen 2014: 6–9. —? Duffy et al. 2016a: 1691–1692, 1694–1696. —? Duffy et al. 2016b: 424. —? Lacey et al. 2016: 126, 128, 131. — Corbari & Sorbe 2018: 2. —? Bribiesca-Contreras et al. 2021: 9.
Orchomenopsis (Orchomene) abyssorum – Costello et al. 1989: 32 (in list).
Orchomene (Abyssorchomene) abyssorum –? Barnard & Ingram 1990: 26–29, figs 15–17.
non Orchomenopsis chilensis View in CoL f. abyssorum – Schellenberg 1926: 291–292, fig. 27 (= Abyssorchomene scotianensis View in CoL ).
non Orchomenella abyssorum – Barnard K.H. 1932: 69, fig. 28 (= Abyssorchomene cf. scotianensis View in CoL , except fig. 27b = Abyssorchomene abyssorum View in CoL ). — Nicholls 1938: 35, fig. 15 (= Abyssorchomene cf. scotianensis View in CoL ). — Dahl 1954: 282 (= Abyssorchomene cf. scotianensis View in CoL ). — Dahl 1959: 225 (= Abyssorchomene View in CoL sp. nov.). — Birstein & Vinogradov 1962: 41 (= Abyssorchomene cf. scotianensis View in CoL ).
non Orchomene abyssorum Arnaud 1974: 572 (ecology), (= Abyssorchomene scotianensis View in CoL ). — Lowry 1982: 320 (= Abyssorchomene scotianensis View in CoL ). — Wakabara et al. 1990: 2, 4, 6 (= Abyssorchomene cf. scotianensis View in CoL ).
non Abyssorchomene abyssorum View in CoL – Thurston 1990: 262–263, 269 (= A. patriciae View in CoL in part; part = A. cf. patriciae View in CoL ; T. Horton, pers. com.). — Diffenthal & Horton 2007: 31 (= A. gerulicorbis View in CoL ; T. Horton, pers. com.). — Horton & Thurston 2009: 433–434 (= A. gerulicorbis View in CoL ; T. Horton, pers. com.). — Gutteridge 2012: 5, 22, 24 (= A. patriciae View in CoL ; T. Horton, pers. com.). — Corrigan et al. 2013: 156, 158–161 (= A. cf. patriciae View in CoL ). — Cousins et al. 2013: 303-304 (= A. cf. shannonae View in CoL ; T. Horton, pers. com.). — Duffy et al. 2013: 360–368 (= A. patriciae View in CoL ; T. Horton, pers. com.). — Horton et al. 2013: 352, 354–358 (= A. patriciae View in CoL ; T. Horton, pers. com.). — Priede et al. 2013: 8 (= A. cf. patriciae View in CoL ). — Horton et al. 2020: 6–7, 11 (= A. patriciae View in CoL ; T. Horton, pers. com.).
New diagnosis
Pereonites 1–7 and pleonites 1–2 with a weak but distinct dorsoposterior hump on each segment. Lateral cephalic lobe broadly rounded, dorsal margin regularly convex and slightly more convex than the nearly straight ventral margin. Antennae 1–2 of male with calceoli, female without. Epistome level with upper lip. Maxilla 1 palp, distal end weakly convex with conical apical spines contiguous. Maxilliped inner plate, distal margin conspicuously excavate; outer plate inner margin not scalloped. Coxa 1 slightly widened distally, ~ 1.2–1.3× proximal width. Gnathopod 2 propodus of male and female narrow, length ~ 2.3–2.5 × width, dactylus very small, inserted on the posterodistal one-third of distal margin, palm lacking concavity. Coxa 5 very weakly posterolobate, posterodistal lobe narrow with posterodistal margin nearly straight. Pereopod 7 basis with anterior margin slightly concave, posterior margin subparallel to anterior margin in the proximal two-thirds, distal third with a distinct, nearly straight bevel (male less distinct and more rounded). Uropod 1 peduncle length greater than 1.5–1.7 × length of outer ramus. Uropod 3 inner ramus extends past distal end of article 1 and reaches ~ 70% length of article 2 of outer ramus. Telson cleft ~ 52–56% of length.
Material examined
Holotype SOUTHWEST ATLANTIC • ♂ (7.5 mm, figured, see Figs 5–9 View Fig View Fig View Fig View Fig View Fig , dissected in 3 tubes, carcass missing/lost); east of Buenos Aires; HMS Challenger, station 323; 35°39′ S, 50°47′ W; depth 3475 m (1900 fathoms); bottom blue mud, temperature 33.1°F; 28 Feb. 1876; BMNH 1889.5.15.23 GoogleMaps
Additional material
SOUTHWEST ATLANTIC • 1 ♀ (9.5 mm, mature, with setose brood plates, illustrated, see Figs 2–4 View Fig View Fig View Fig , appendages on 2 slides); Argentine Basin; RV Meteor DIVA-3 M79/1 expedition, station 531; 35°56.49′ S, 48°53.85′ W; depth 4586 m; gear, baited traps; 15 Jul. 2009; E. Hendrycks leg.; ZMH K-61206 GoogleMaps • 1 ♂ (7.0 mm, appendages on 2 slides, partly illustrated, see Fig. 2 View Fig ); same collection data as for preceding; ZMH K-61207 GoogleMaps • 5 ♂♂, 5 ♀♀, 4 juveniles; same collection data as for preceding; ZMH K-61208 GoogleMaps • 10 ♂♂, (one of these 7.0 mm, dissected, appendages on 3 slides), 5 ♀♀, 7 juveniles; same collection data as for preceding; CMNC 2022-0001 GoogleMaps • 5 ♂♂, 10 ♀♀, 3 juveniles; same collection data as for preceding; RBINS INV. 138.486 GoogleMaps .
NORTH ATLANTIC • 9 ♀♀ (with mature ♀♀, 7–10 mm, 1 mature ♀, 10 mm, appendages on 8 slides, 1 nearly mature ♀, 9.5 mm, appendages on 3 slides); near Azores; Hirondelle 1896 campaign, station 730; 37°58′ N, 28°33′30″W; depth 2660 m; bottom sandy clay; gear, baited traps; 3–5 Aug. 1896; MNHN, collection Chevreux (no registration number, see Chevreux 1903: 92) GoogleMaps .
Description
Female
Based on female (mature), 9.5 mm (ZMH K-61206).
PEREONITES 1–7 AND PLEONITES 1–2 ( Fig. 2 View Fig ). With weak but distinct dorsoposterior hump on each segment.
PLEONITE 3 ( Fig. 2 View Fig ). With a distinct, rounded posterodorsal elevation slightly overhanging urosomite 1.
COXAE 1–2 ( Figs 2–3 View Fig View Fig ). Subequal to slightly longer (1.1 × to 1.25 ×) than the depth of corresponding pereonites (in lateral view).
COXAE 3–4 ( Fig. 4 View Fig ). Slightly longer (1.1 × to 1.25 ×) than the depth of corresponding pereonites, coxa 3 not shown.
EPIMERON 3 ( Fig. 2 View Fig ). Subquadrate, with posterodistal angle slightly obtuse, apex rounded, posterior margin weakly but regularly convex, ventral margin regularly convex.
UROSOMITE 1 ( Fig. 2 View Fig ). With a deep, narrow dorsal concavity in front of the strong, regularly rounded dorsal boss, convex on posterior margin, boss projecting strongly upright and backward, slightly overhanging urosomite 2.
HEAD ( Fig. 2 View Fig ). About equal in length to pereonite 1.
LATERAL CEPHALIC LOBE ( Fig. 2 View Fig ). Broadly rounded; dorsal margin regularly convex and slightly more convex than the nearly straight ventral margin.
EYE ( Fig. 2 View Fig ). Nearly indistinguishable, but non ommatidial, formed of pigment granules; crescent-shaped, narrow, parallel to head anterior margin (estimated in figure by dotted outline).
ANTENNA 1 ( Fig. 2 View Fig ). Peduncular article 1 without anterodistal lobe, slightly dilated, length about 1.4× width; flagellum 10-articulate, first article of flagellum callynophorate, densely furnished with double row of aesthetascs medially, callynophore small, shorter (67%) than the remaining articles combined; accessory flagellum 5-articulate, first article slightly shorter (80%) than remaining articles combined; calceoli absent.
ANTENNA 2 ( Fig. 2 View Fig ). Slightly longer than antenna 1; geniculate between peduncular articles 3–4, peduncular articles 4–5 lined with anteromedial brush setae; flagellum 12-articulate, calceoli absent.
EPISTOME ( Fig 2 View Fig ). Level with the upper lip, from which it is separated by a small slit and weak shallow indentation; weakly concave above upper lip.
UPPER LIP ( Fig. 2 View Fig ). Anterior margin convex, not protruding; midventral margin with a small, angular projection.
MANDIBLE. Incisor distinctly convex and slightly widened, dorsolateral and ventromedial corners with small tooth; left lacinia mobilis slender, curved, distally with small teeth, right lacking; accessory spine row with 3 strong spines, interspersed with fine setae; molar somewhat falciform, not columnar, forming a narrow crest, acutely produced on proximal end, setose with small triturative surface, hairy process (cf. Oleröd 1975) located proximal to molar; palp attached proximal to molar; article 2 with 15A2 setae, article 3 missing (refer to Fig. 6 View Fig of male holotype for general morphology and setae details).
LOWER LIP. Outer lobes broad, inner margins strongly setose, distal inner margins excavated; without inner lobes; mandibular processes rounded.
MAXILLA 1 ( Fig. 2 View Fig ). Outer plate with 11 spine-teeth in 7/4 crown arrangement; palp article 2 widened, with 9 contiguous (or nearly contiguous, separated by much less than the width of a spine) distal conical spines and one apical strong seta on outer corner; inner plate short with subrounded apical projection, and two unequally sized plumose setae inserted subapically (refer to Fig. 7 View Fig of male holotype for general morphology).
MAXILLA 2. Outer and inner plates strongly tapering distally, both lined with strong rows of pectinate medial marginal spines and setae; inner plate much shorter and slightly narrower than outer plate, apex just reaching the proximal end of medial setal row of outer plate (refer to Fig. 7 View Fig of male holotype for general morphology).
MAXILLIPED ( Fig. 2 View Fig ). Inner plate short, reaching about one third length of outer plate and reaching half length of palp article 1; outer corner of inner plate reaching about the level of the basal insertion of palp article 2; inner plate, distal margin conspicuously excavate, with slight mediodistal extension protruding slightly higher than the outer corner, with 3 weakly protruding nodular spines, unequally spaced, the two inner nodular spines closer to each other, the third nodular spine at the bottom of the excavation; outer plate well developed, subovate, length 1.8× width, distal margin of outer plate reaching slightly below the distal margin of palp article 2, with two dissimilar apical spines and numerous (~ 12) strongly embedded, medial nodular spines, medial margin nearly smooth; palp strongly setose medially, article 4 well developed, about 60% of the length of article 3, with few apical fine setae on inner margin (refer to Fig. 7 View Fig of male holotype for general morphology).
GNATHOPOD 1 ( Fig. 3 View Fig ). Coxa moderately widened, distal width 1.3× proximal width and about 72% of length; anterior margin weakly concave; posterior margin very slightly concave, nearly straight; distal margin slightly convex in posterior half, more strongly convex in anterior half; basis stout, as wide as propodus, anterior margin with 7 very short setae; ischium subequal to merus; carpus short, less than half the length of propodus, posterodistal lobe not guarding propodus; propodus subchelate, subrectangular, very weakly tapering, length 1.57 × width, posterior margin slightly concave with distinct inflexion point at about distal two-thirds; palm slightly convex, with irregular microserrations, palm corner with 2 blunt protrusions and defined by 1 medial and 1 lateral spine; dactylus barely overriding palm corner.
GNATHOPOD 2 ( Fig. 3 View Fig ). Coxa subrectangular, length 2.36 × width, anterior margin nearly straight, posterior margin slightly concave, distal margin straight; ischium shorter than carpus; carpus length 1.8× propodus; propodus chelate, surface finely setose with distal groups of long pectinate setae, slender, length 2.45 ×width, much narrower (0.6 ×) than carpus, ventral margin very slightly concave, nearly straight; dactylus very small, inserted on the posterodistal one-third of distal margin, with distal microornamentation, palm not excavate.
PEREOPOD 3. Coxa subrectangular, with anterior margin nearly straight, posterior margin slightly concave, length 2.4 × width; rest of pereopod as in pereopod 4.
PEREOPOD 4 ( Fig. 4 View Fig ). Coxa length 1.38 × width, posterior margin strongly excavate, with strong, subtriangular posterodistal lobe, angle subquadrate and apex rounded, posterior corner located at distal 66% of the length; posterior margins of ischium-merus-carpus with clusters of long setae; propodus with 6 short spine groups; dactylus 0.44× length of propodus.
PEREOPOD 5 ( Fig. 4 View Fig ). Coxa slightly wider (1.15 ×) than long, very slightly posterolobate, posterodistal lobe narrow, not regularly convex, with posterodistal margin nearly straight; basis shorter than coxa, length 0.84× coxa, length 1.12 × width, posterodistal lobe broadly rounded, slightly surpassing the distal margin of ischium; merus slightly expanded, width 0.7 × length, bearing anterior and posterior setae; rest of pereopod missing.
PEREOPOD 6. Basis weakly narrowing distally, with posterior margin slightly serrate and regularly convex, anterior margin nearly straight on 80% of its length, length 0.70 × the combined length of the remaining articles, posterodistal lobe not reaching distal margin of ischium; merus slightly expanded (narrower than in P5) and bearing few anterior and posterior setae; propodus slightly shorter than merus-carpus, with 6 clusters of short spines; dactylus about 0.3× length of propodus.
PEREOPOD 7 ( Fig. 4 View Fig ). Coxa small, subovate; basis with anterior margin slightly concave, posterior margin subparallel to anterior margin in the proximal two-thirds, distal third with a distinct, nearly straight bevel; posterior margin with 10–11 weak serrations; posterodistal lobe not extending to distal margin of ischium; merus not expanded (narrower than P6), anterior margins of merus-carpus with short spine groups, posterior margin bearing few short slender spines; propodus slightly shorter than merus-carpus; dactylus 0.35 × length of propodus.
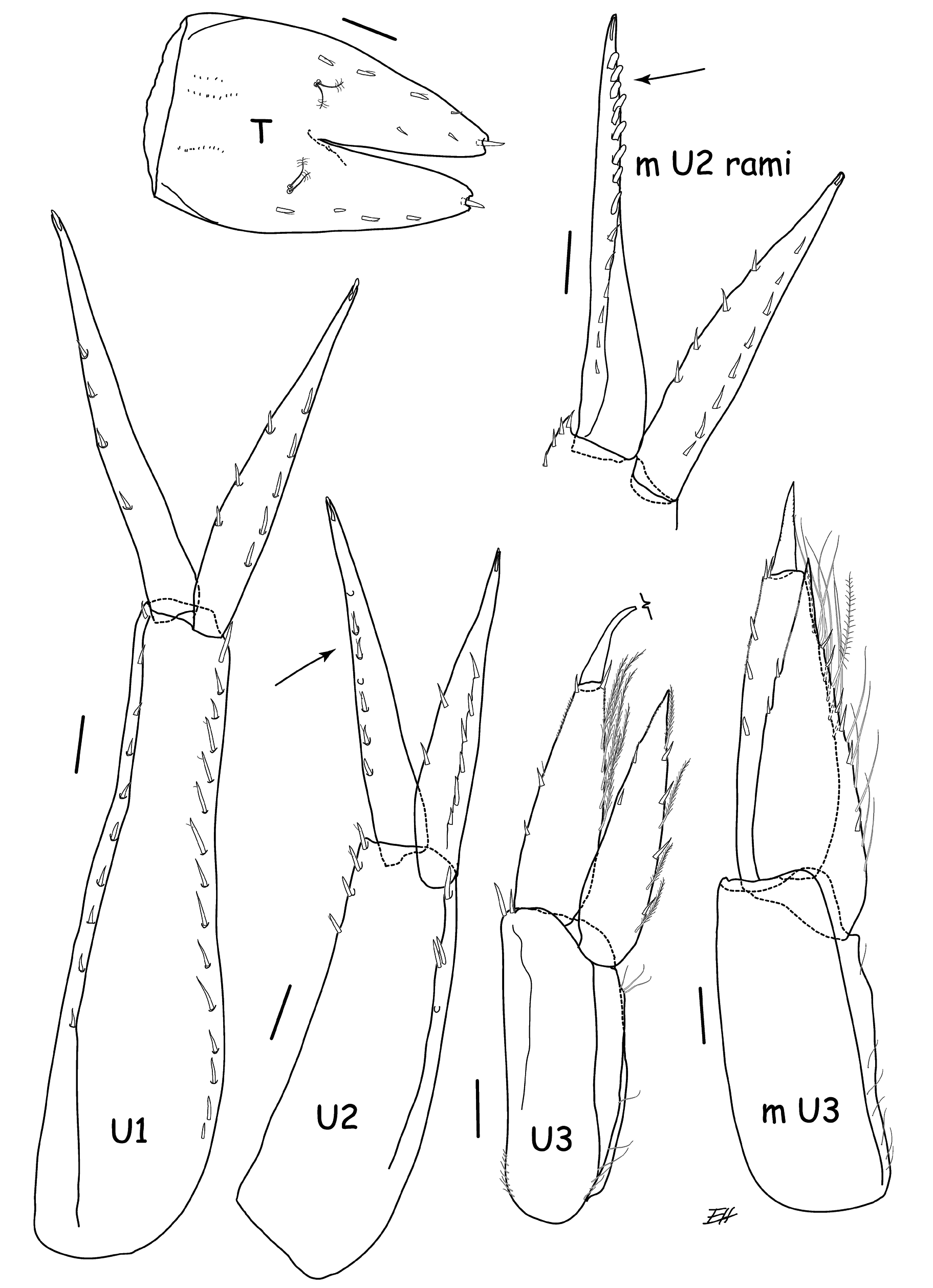
UROPOD 1. Peduncle relatively long, about 1.5× length of outer ramus and 1.7× length of inner ramus, dorsolateral and dorsomedial margins spinose (with 14 dorsomedial spines); inner ramus slightly shorter than outer, margins of rami poorly spinose, with slender spines, medial margin of outer ramus lacking spines (refer to Fig. 9 View Fig of male holotype for general morphology).
UROPOD 2 ( Fig. 2 View Fig ). Peduncle 1.27 × length of outer ramus, with 5 closely spaced spines on distal outer margin, inner margin with 3; inner ramus shorter than outer ramus; outer ramus with 6 equally spaced thin sharp spines on dorsolateral margin; inner ramus margins with small slender spines.
UROPOD 3 ( Fig. 2 View Fig ). Peduncle 0.79 × length of biarticulate outer ramus; second article of outer ramus long, about 0.5 × length of article 1; inner ramus extends past distal end of article 1 and reaches ~ 70% of article 2 of outer ramus; inner margins of rami with long plumose setae and few spines.
TELSON ( Fig. 4 View Fig ). 1.6 × longer than wide, cleft (52.5%), lobes tapering distally with 4–5 lateral, submarginal spines and 1 distal spine.
GILLS 5–6. With 1 long tubular accessory lobe on gill 5 and 2 on gill 6, inserted basally.
GILL 7 ( Fig. 4 View Fig ). Present, well developed.
BROOD PLATES ( Figs 3–4 View Fig View Fig ). On gnathopod 2 and pereopods 3–5, long, slender and curved distally, largest on gnathopod 2 and pereopods 3–4, with long brood setae, 16 setae on gnathopod 2 plate and 15 on pereopod 4.
STOMODEUM. Extending to the 7 th pereonite.
Male
Based mostly on 7.5 mm holotype, Figs 5–9 View Fig View Fig View Fig View Fig View Fig (BMNH 1889.5.15.23) and 7 mm male (ZMH K-61207) specimens ( Fig. 2 View Fig for head and body characteristics).
Similar to female, but differing as follows:
BODY. Smaller than female and slightly less robust.
LATERAL HEAD LOBE ( Fig. 2 View Fig ). Slightly narrower.
ANTENNA 1 ( Figs 2 View Fig , 5 View Fig ). Peduncular article 1 stouter, more compact; callynophore stronger, about as long or slightly longer than the remaining flagellar articles, flagellum 12–13-articulate, articles shorter, slightly setose, with calceoli.
ANTENNA 2 ( Figs 2 View Fig , 5 View Fig ). Slightly longer (1.25×) than antenna 1; flagellum 13–16-articulate, with calceoli.
COXA 1 ( Figs 2 View Fig , 8 View Fig ). Weakly widened distally, less than in female, distal width 1.25 × proximal width and 60% length, anterior margin and posterior margins very slightly concave at midpoint.
COXA 2–4 ( Fig. 9 View Fig ). Distinctly narrower than in female, coxa 4 length 1.5 × width.
MANDIBLE ( Fig. 6 View Fig ). Incisor distinctly convex and slightly widened, dorsolateral and ventromedial corners with small tooth; left lacinia mobilis curved, with 2 strong and 1 weak apical teeth, right lacking; accessory spine row with 3 strong spines, interspersed with fine setae; molar forming a narrow crest, somewhat falciform, acutely produced on proximal end, setose with small triturative surface; hairy process (cf. Oleröd 1975) located proximal to molar; palp attached proximal to molar; article 2 1.58× length of article 3, with 20 A2 setae, article 3 straight, slightly falciform, with 1 A3 seta, a long rank of 20 D3- pectinate marginal setae and 2 E3 seta.
LOWER LIP. Outer lobes broad, inner margins strongly setose, distal inner margins excavated; without inner lobes; mandibular processes rounded.
MAXILLA 1 ( Fig. 7 View Fig ). Outer plate with 11 spine-teeth in 7/4 crown arrangement; palp article 2 very weakly widened, with 8 distal conical spines and one apical strong seta on outer corner; inner plate with short subacute apical projection, and two unequally sized plumose setae inserted subapically.
MAXILLA 2 ( Fig. 7 View Fig ). Outer and inner plates strongly tapering distally, both with strong rows of pectinate medial marginal spines and setae; inner plate much shorter and narrower than outer plate (narrower than female), apex just reaching the proximal end of medial setal row of outer plate.
MAXILLIPED ( Fig. 7 View Fig ). Inner plate short, reaching about 0.3× length of outer plate and reaching half length of palp article 1; outer corner of inner plate reaching about the level of the basal insertion of palp article 2; inner plate, distal margin conspicuously excavate, slight mediodistal extension about equal to outer corner (lower than female), with 3 weakly protruding nodular spines, unequally spaced, the two inner nodular spines closer to each other, the third nodular spine at the bottom of the excavation; outer plate well developed, subovate, length 1.8× width, distal margin of outer plate slightly shorter than distal margin of palp article 2, with two dissimilar apical spines and numerous (9–10) strongly embedded, medial nodular spines, medial margin nearly smooth; palp strongly setose medially, article 4 well developed, about 60% of the length of article 3, with 3 apical setae on inner margin (note: medial marginal setae omitted on palp of Mxpd).
GNATHOPOD 1 ( Fig. 8 View Fig ). Basis narrower than propodus width, anterior margin with only 1 short seta; propodus of similar proportions to female, length 1.55× width, but posterior margin slightly less concave.
GNATHOPOD 2 ( Fig. 8 View Fig ). Propodus of similar shape and proportions to female, length ~ 2.3 × width.
PEREOPOD 5 ( Fig. 9 View Fig ). Coxa slightly more posterolobate; basis slightly longer, length 1.17 × width.
PEREOPOD 7 ( Fig. 9 View Fig ). Basis, posteroventral bevel not as distinct or as straight.
UROPOD 1 ( Fig. 9 View Fig ). Peduncle long, about 1.7 × length of outer ramus and 1.9 × length of inner ramus, dorsolateral and dorsomedial margins spinose; inner ramus shorter than outer, margins of rami poorly spinose, with slender spines, medial margin of outer ramus lacking spines.
UROPOD 2 ( Figs 2 View Fig , 9 View Fig ). Distolateral marginal spines of peduncle grouped closely together at distal end of peduncle; inner ramus distinctly shorter than outer; outer ramus distolateral spines stouter, more numerous, bluntly rounded and more closely spaced distally, proximal spine acute (see comments, p. 24).
UROPOD 3 ( Fig. 9 View Fig ). Second article of outer ramus shorter than female, about 0.34 × length of article 1.
UROSOMITE 1 ( Fig. 2 View Fig ). Dorsal concavity broader, boss slightly narrower, posterior margin less convex.
TELSON ( Fig. 9 View Fig ). Cleft deeper (56%) than female.
Distribution
Southwest Atlantic: Argentine Abyssal Basin ( Stebbing 1888; this paper), Northeast Atlantic abyssal plains ( Chevreux 1903). Pacific deep-sea ( Dahl 1959; Birstein & Vinogradov 1960; Barnard & Ingram 1990; Lowry & Stoddart 1994; Lacey et al. 2016) and Indian Ocean occurrences ( Birstein & Vinogradov 1964; Witte 1999; Janssen et al. 2000) remain to be confirmed. No co-occurrence with A. patriciae sp. nov. was documented so far.
Depth range
Bottom records: 3475 m ( Stebbing 1888) to 4586 m (this paper). Midwater records: 2178 m from the surface ( Chevreux 1903). Along with A. scotianensis and A. shannonae sp. nov. (see below), A. abyssorum is one of many scavenging amphipods recorded so far by midwater trawls at some distance from the bottom. Other documented cases include A. chevreuxi ( Stebbing, 1906) ; A.? distinctus ( Birstein & Vinogradov, 1960) ; Cyclocaris sp. ; Eurythenes sp. ; Paralicella caperesca Shulenberger & Barnard, 1976 ; P. tenuipes Chevreux, 1908 ; Scopelocheirus sp. and Valettieta gracilis Lincoln & Thurston, 1983 : see Thurston 1990. As well as: Pseudorchomene coatsi (Chilton, 1912) ; A. plebs (Hurley, 1965) ; A. rossi ( Walker, 1903) and Orchomenella cavimanus ( Stebbing, 1888) : see De Broyer et al. 2007.
Remarks
Orchomene abyssorum was described by Stebbing (1888), from a single male specimen (about 7.5 mm long) collected by the HMS Challenger in the Argentine Basin at a depth of 3475 m. As shown by the extensive citation list, Abyssorchomene abyssorum has been widely reported and is generally considered as having a wide-ranging, even cosmopolitan distribution ( Barnard & Ingram 1990; Thurston 1990, 2001; Barnard & Karaman 1991; Duffy et al. 2013), but this concept is being challenged and will change with our study. There are still many questions regarding species identity of single specimens which await confirmation by critical study of the material.
We examined and refigured the male holotype specimen (appendages and mouthparts; the carcass is apparently lost). As a result, we have noticed some obvious discrepancies between our observations and drawings, and the original illustrations of Stebbing. Some of these are as follows (Stebbing’s differences are outlined in parentheses): antenna 1 peduncular article 1 dorsal margin is less convex and the ventral margin is less concave (vs dorsal margin strongly convex, ventral margin strongly concave); mandible palp article 3 is straighter (vs strongly curved and more falciform); maxilla 1 palp is shorter, less curved with the distal margin straighter (vs palp longer, more strongly curved with distal margin strongly convex); maxilla 2 outer plate broader (1.35 ×), (vs very narrowed, much less than width of inner plate); maxilliped outer plate shorter, with inner margin nearly straight (vs outer plate longer, with inner margin concave and strongly serrated); gnathopod 1 propodus is broader (length 1.5× width) with a short carpal lobe (vs longer, length 2 × width and more curved, with a longer carpal lobe, strongly guarding the hind margin); gnathopod 2 propodus has a very slight concave ventral margin, with a small dactylus and the posterodistal margin is slightly convex (vs strongly concave ventral margin, with a larger dactylus and the posterodistal margin is strongly sloped, level with the dactylus); epimeron 3 is narrowly rounded at the posteroventral corner (vs more broadly rounded at the posteroventral corner) and the dorsal boss of urosomite 1 is narrowly rounded and more upright (vs broadly rounded and lower). There are other minor differences which we have not included here. Reasons for these discrepancies are most likely numerous but possibly related to microscopy drawing technique and available equipment, attention to various details and whether these were completed freehand in some cases.
A special dimorphic character has been found in the male uropod 2 outer ramus distolateral spines. They are in a comb-like arrangement and are much stouter and bluntly rounded, more numerous and more closely spaced distally, with the proximal spine(s) being acute. This morphology and arrangement of ramal spines are not found in females, which are always acute, slender and more evenly spaced. The function of this peculiar spine morphology and arrangement in males are unknown, but possibly related to mating. All male specimens of Abyssorchomene discussed present this uropod 2 outer ramus spine character and it has not been reported or elaborated in the literature as far as we know (see Figs 2 View Fig , 9 View Fig , 17 View Fig , 25 View Fig , 33 View Fig ).
Part of the material identified by Chevreux (1903) from the Hirondelle campaign 1896 (station 730 near the Azores, depth 2660 m) was also examined. It consisted of nine female specimens including fully mature ones. These specimens present all the diagnostic characters of Abyssorchomene abyssorum as redescribed here (in particular, the weak dorsal corrugations – sometimes difficult to distinguish – and the narrow female gnathopod 2 propod without an excavate palm), confirming Chevreux’s identification. The rest of Chevreux’s material (1935) collected from some other North Atlantic abyssal regions has not been seen and remains to be carefully checked.
Walker (1903) recorded three specimens of which one is registered in the Natural History Museum (London) collections: BMNH 1905.9.7.23: one specimen, 6 mm, non-gravid, without visible oostegites, male (?), with gnathopod 2 propod length more than twice the width and palm straight, without a proximal concavity (Lauren Hughes, pers. com.), which does not allow to differentiate it from the male of A. patriciae sp. nov. In his paper, Walker did specifically thank Stebbing for verification of his Orchomene abyssorum identification, but we consider this identification as uncertain.
We also checked the single specimen identified by Stephensen (1925) from the Ingolf 1895–96, station 91, North Atlantic, west of Iceland, 64°44′ N, 31°00′ W, depth 2317 m, bottom 3.1°C, held in the Zoological Museum, University of Copenhagen (ZMUC). The specimen is fragmentary and in poor condition, with the head missing. We recognise it as a male. The slender gnathopod 2 propodus was used by Stephensen to attribute the specimen to Orchomenopsis abyssorum , but this male character is shared by all species of the A. abyssorum complex. So, due to the poor and incomplete condition of this specimen, the definitive identification remains unclear.
Dahl (1959) discussed a 12 mm female specimen from the Galathea station 649, southwest Pacific, Kermadec Trench, 35°16′ S, 178°40′ W, depth 8210–8300 m, grey clay with pumice, 14 February 1952, also held in the ZMUC collections, but unfortunately provided no figures. We examined his material and found that it differs from A. abyssorum by the more strongly expanded coxa 1, the proportionally shorter propodus of gnathopod 1, the wider, subovate propodus of gnathopod 2 and the strongly convex distal margin of maxilla 1 palp. As well, this material comes from much greater depths than A. abyssorum . We conclude that it is possibly a new species of Abyssorchomene .
From the figures and comments provided by Birstein & Vinogradov (1960), including the first detailed reference to a female (from the Kermadec Trench), we are of the opinion that their specimens differ in many characters from A. abyssorum as redescribed here. We have not seen this material. The figures show that these specimens differ in the following ways: in the more elongate carpal lobe of gnathopod 1 slightly but distinctly guarding the propodus, the proportionally smaller and narrower male gnathopod 1 propodus, the more elongate carpus of gnathopod 2, the distinctly curved propodus of gnathopod 2, the slightly spaced terminal spines on the maxilla 1 palp, the slightly different shape of the pereopod 7 basis with the hind margin parallel to the anterior margin on two-thirds of its length, the shorter inner ramus of uropod 3 and the sinuous inner margins of the telson lobes. Contrary to Barnard & Ingram (1990), we doubt that these morphological differences in the Kermadec Trench specimens can be considered as intraspecific variations of A. abyssorum . Until these specimens are examined in detail, the species attribution remains unknown.
Birstein & Vinogradov (1964) collected, in the Central Indian Ocean, a male (8 mm) that we cannot attribute with certainty to A. abyssorum , as there were no illustrations of the specimen and we were not able to examine the material to confirm its identity.
Barnard & Ingram (1990) redescribed in detail A. abyssorum based on new material (a single small male, 6.41 mm, Scripps Institution of Oceanography ( SIO ), station 882, Galapagos Vents area, 00°47.9′ N, 86°09.2′ W, depth 2491 m). They considered Chevreux’s (1900, 1903, 1935) North Atlantic material, as well as Birstein & Vinogradov's (1960) records as the only firm identifications of A. abyssorum . The small male described and finely illustrated shows the gnathopod 1 carpal lobe slightly more elongate and guarding the propodus to a greater degree than on the type and DIVA- 3 specimens. The pereopod 5 basis has a slightly different relative length: it is shorter than the coxa instead of longer in the type specimen and the DIVA- 3 males. This shape is closer to that of the DIVA- 3 female described here and may be due to the immature state of the specimen. The epimeron 3 shows a slightly sinuous posterior margin which is very slightly and regularly convex, but nearly straight in the type, DIVA-3 and Chevreux’s material. Like Stebbing (1888), Barnard & Ingram (1990) did not mention the presence of the weak dorsal corrugations, clearly distinct on our DIVA-3 material and slightly less so in Stebbing’s figure of pleonites 1–2, but not on the pereonites on his in-toto illustration. Other minor differences between the male described by Barnard & Ingram (1990) and the type and DIVA- 3 male specimens include: the shorter extension of the poorly distinct eye, i.e., 44% of the head height instead of 75% (a character however unclear and somewhat unreliable in long preserved specimens); the much narrower lateral head lobe; the coxa 4, with a very different shape and smaller posteroventral lobe; the coxa 5 width subequal to its height (instead of slightly wider than high); the much shorter inner ramus of uropod 3; the maxilla 2 with tip of inner plate not reaching the basal end of outer plate setal row and the nodulous apical spines of the maxilliped inner plate located differently. Given all these differing characters, it is unlikely to belong to A. abyssorum and at this time the specific attribution of this single male specimen remains unresolved.
Thurston (1990) collected A. abyssorum in four North Atlantic abyssal plains by bottom traps or midwater trawls but didn’t make any morphological comment. It is important to note that not all the specimens in this paper were seen by Thurston (Tammy Horton, pers. com.). However, T. Horton (pers. com.) checked the sample from the Porcupine Abyssal Plain (see Table 1 View Table 1 ) and concluded it belongs to A. patriciae sp. nov. This likely applies to all the samples examined by Thurston 1990.
Concerning the Southern Ocean material attributed to A. abyssorum ( Schellenberg 1926; Barnard 1932; Nicholls 1938; Dahl 1954), Dahl (1959) expressed his doubts about its conspecifity with A. abyssorum ( Stebbing 1888) . Andres (1983) definitively distinguished the Antarctic continental shelf specimens from the abyssal and hadal ones in describing A. scotianensis . He considered the juvenile specimens of Schellenberg (1926) as belonging to his new species but couldn’t confirm the affiliation of the specimens of Barnard (1932), Nicholls (1938) and Dahl (1954), as the length-width relationship of coxa 1 and of the basis-propodus of gnathopod 1 were not described.
All A. abyssorum identifications should be carefully checked by considering the new species described here. In Table 1 View Table 1 , we tentatively attribute the various records to the most likely correct species determined by our study.
Abyssorchomene abyssorum shares with the two new species, A. patriciae sp. nov. and A. shannonae sp. nov. dorsodistal corrugations on the pereonites 1–7 and pleonites 1–2, a character unique among all the species of the genus Abyssorchomene (sensu Lowry & Kilgallen 2014; Horton et al. 2021). This character separates this complex from the remaining Abyssorchomene species. All three species are very close morphologically and males are very difficult to separate.
In A. abyssorum , the gnathopod 2 propod, similar in both sexes, is very slender (length 2.3–2.5 × width) and the palm presents a very narrow gap and lacks a concavity. In contrast, the females of A. patriciae sp. nov. and A. shannonae sp. nov. have a broadened, suboval propod (length 1.5–1.7× width) with a very distinct concavity on the palm. Coxa 1 is slightly (females) to weakly (males) widened distally. It is slightly more widened in both the new species, A. patriciae and A. shannonae (especially in females). Coxa 5 is very weakly posterolobate in A. abyssorum but distinctly more in A. patriciae and A. shannonae . The distal bevel of the posterior margin of the pereopod 7 basis is straighter in A. patriciae and A. shannonae , and in the former species, the basis is more subrectangular, with the front margin straight. The uropod 3 inner ramus extends to about 70% of the length of the outer ramus article 2; in A. shannonae , it is much shorter, reaching about 20–25% of the length of the outer ramus article 2 and in A. patriciae , the inner ramus reaches to about 63% of the length of article 2. Other differences are provided in the key to the species (see p. 68).
Table 1 (continued on next four pages). Overview of Abyssorchomene abyssorum (Stebbing, 1888) (s.l.) specimen records in literature. (Abbreviations: [fide TH] = T. Horton, pers. com.; mab = m above bottom; fm = fathom).
| Source | Original ID | Specimens | Attributed to | Ocean | Location | Expedition-ship-year-station | Lat./Long. (DMS) | Min./Max. mab depth (m) |
|---|---|---|---|---|---|---|---|---|
| Stebbing 1888 | Orchomene abyssorum | 1 ♂ | Abyssorchomene abyssorum | SW Atlantic | Argentine Basin | Challenger (1873–76) stn 323 | 35°39′S, 050°47′W | -/3475 (1900 fm) |
| Chevreux 1900 | Orchomenopsis abyssorum | 1 ♂ | A.? abyssorum | NE Atlantic | W of Porcupine Abyssal Plain | Hirondelle (1888) stn 256 | 48°24′48″N, 020°38′30″ W | 2200 / |
| Chevreux 1903 | O. abyssorum | 2 ♀ | A. abyssorum | NE Atlantic | W of Azores Is. | Princesse Alice I (1895) stn 532 | 37°52′N, 027°03′W | -/2178 |
| Chevreux 1903 | O. abyssorum | many ind. | A. abyssorum | NE Atlantic | W of Azores Is. | Princesse Alice I (1896) stn 730 | 37°58′N, 028°33′30″ W | -/2660 |
| Walker 1903 | O. abyssorum | 1 ind. | A.? abyssorum | NE Atlantic | N of Porcupine Abyssal Plain | Oceana (1898) tow-net 4j | 52°27′06″N, 015°40′ W | -/2871 (1570 fm) |
| Walker 1903 | O. abyssorum | 1 ind. | A.? abyssorum | NE Atlantic | N of Porcupine Abyssal Plain | Oceana (1898) tow-net 5h | 52°18′01″N, 015°53.9′W | -/2578 (1410 fm) |
| Walker 1903 | O. abyssorum | 1 ind. | A.? abyssorum | NE Atlantic | N of Porcupine Abyssal Plain | Oceana (1898) tow-net 5k | 52°18′01″N, 015°53.9′W | -/2761 (1510 fm) |
| Stephensen 1925 | O. abyssorum | 1 ♂ | A. sp. | NE Atlantic | W of Iceland | Ingolf (1895 – 96) stn 91 | 64°44′N, 031°00′W | -/2317 |
| Schellenberg 1926 | O. chilensis f. abyssorum | 3 juv. | A. scotianensis | Southern Ocean | Wilhelm II Coast | Gauss (1901–03) Gauss-station | 66°02′S, 089°38′E | -/385 |
| Barnard 1932 | Orchomenella abyssorum | 1♂, 1 ♀ | A. cf. scotianensis | Southern Ocean | off Livingston I. | Discovery (1925 – 27) stn 208 | 62°49′30″S, 060°10′30″ W | 0/800 (787) |
| Chevreux 1935 | Orchomenopsis abyssorum | see Chevreux 1903 | A. abyssorum | NE Atlantic | W of Azores Is. | See Chevreux 1903 | ||
| Chevreux 1935 | O. abyssorum | 3 ind. | A. cf. abyssorum | NE Atlantic | Biscay Abyssal Plain | Princesse Alice II (1903) stn 1479 | 44°39′N, 002°11′W | -/1414 |
| Chevreux 1935 | O. abyssorum | 1 ind. | A. cf. abyssorum | NE Atlantic | Biscay Abyssal Plain | Princesse Alice II (1903) stn 1500 | 44°34.0′ N, 004°38.5′ W | -/4330 |
| Chevreux 1935 | O. abyssorum | 1 ind. | A. cf. abyssorum | NE Atlantic | N of Canary Is. | Princesse Alice II (1904) stn 1760 | 29°16′N, 016°11′W | 0/3000 |
| Chevreux 1935 | O. abyssorum | 3 ind. | A. cf. abyssorum | NE Atlantic | SW of Azores Is. | Princesse Alice II (1904) stn 1856 | 36°46′N, 026°41′W | 0/3250 |
| MNHN |
France, Paris, Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
SubPhylum |
Crustacea |
|
Class |
|
|
Order |
|
|
SuperFamily |
Lysianassoidea |
|
Family |
|
|
Genus |
Abyssorchomene abyssorum ( Stebbing, 1888 )
| Hendrycks, Ed A. & Broyer, Claude De 2022 |
Orchomene (Abyssorchomene) abyssorum
| Barnard J. L. & Ingram C. 1990: 26 |
Abyssorchomene abyssorum
| Horton 2020: 6 - 7 |
| Cousins 2013: 303 - 304 |
| Duffy 2013: 360 - 368 |
| Horton 2013: 352 |
| Priede 2013: 8 |
| Gutteridge 2012: 5 |
| Horton & Thurston 2009: 433 - 434 |
| Diffenthal & Horton 2007: 31 |
| Thurston 1990: 262 - 263 |
Orchomenopsis (Orchomene) abyssorum
| Costello M. J. & Holmes J. M. C. & McGrath D. & Myers A. A. 1989: 32 |
Abyssorchomene abyssorum
| Bribiesca-Contreras G. T. G. & Horton T. & Drazen J. C. & Drennan R. & Jones D. O. B. & Leitner A. B. & McQuaid K. A. & Smith C. R. & Taboada S. & Wiklund H. & Glover A. G. 2021: 9 |
| Corbari L. & Sorbe J. C. 2018: 2 |
| Duffy G. A. & Gutteridge Z. R. S. & Thurston M. H. & Horton T. 2016: 1691 |
| Duffy G. A. & Lawler S. F. & Horton T. 2016: 424 |
| Lacey N. C. & Rowden A. A. & Clark M. R. & Kilgallen N. M. & Linley T. D. & Mayor D. J. & Jamieson A. J. 2016: 126 |
| Lowry J. K. & Kilgallen N. M. 2014: 6 |
| Brandt A. & Blazewicz-Paszkowycz M. & Bamber R. N. & Muhlenhardt-Siegel U. & Malyutina M. V. & Kaiser S. & De Broyer C. & Havermans C. 2012: 144 |
| Horton T. 2005: 1 |
| Treude T. & Janssen F. & Queisser W. & Witte U. 2002: 1284 |
| Thurston M. H. 2001: 358 |
| Janssen F. & Treude T. & Witte U. 2000: 3005 |
| Witte U. 1999: 143 |
| Jones E. G. & Collins M. A. & Bagley P. M. & Addison S. & Priede I. G. 1998: 1124 |
| Palerud R. & Vader W. 1991: 32 |
| De Broyer C. 1983: 142 |
Orchomene abyssorum
| Bellan-Santini D. 1998: 145 |
| Kaufmann R. S. 1992: 1 |
| Barnard J. L. & Karaman G. S. 1991: 508 |
| Austin W. C. 1985: 601 |
| Andres H. G. 1983: 209 |
| Lowry J. K. & Bullock S. 1976: 94 |
| Shulenberger E. & Barnard J. L. 1976: 248 |
Orchomene abyssorum
| Wakabara Y. & Tararam A. S. & Valerio-Berardo M. T. & Ogihara R. M. 1990: 2 |
| Lowry J. K. 1982: 320 |
| Arnaud P. M. 1974: 572 |
Orchomenella abyssorum
| Vinogradov G. M. 1999: 1147 |
| Lowry J. K. & Stoddart H. E. 1994: 129 |
| Sanderson J. M. 1973: 37 |
| Thurston M. H. & Allen E. 1969: 364 |
| Birstein Y. A. & Vinogradov M. E. 1964: 164 |
| Hurley D. E. 1963: 125 |
| Gurjanova E. F. 1962: 433 |
| Birstein Y. A. & Vinogradov M. E. 1960: 188 |
| Schellenberg A. 1955: 192 |
| Ruffo S. 1949: 10 |
Orchomenella abyssorum
| Birstein Y. A. & Vinogradov M. E. 1962: 41 |
| Dahl E. 1959: 225 |
| Dahl E. 1954: 282 |
| Nicholls G. E. 1938: 35 |
| Barnard K. H. 1932: 69 |
Orchomenopsis chilensis
| Schellenberg A. 1926: 291 |
Orchomenopsis abyssorum
| Chevreux E. 1935: 59 |
| Stephensen K. 1925: 125 |
| Stebbing T. R. R. 1906: 84 |
| Chevreux E. 1905: 7 |
| Chevreux E. 1903: 92 |
| Walker A. O. 1903: 224 |
| Chevreux E. 1900: 23 |
Anonyx abyssorum
| Della Valle A. 1893: 824 |
Orchomene abyssorum
| Stebbing T. R. R. 1888: 679 |