Kujdanowiaspis podolica ( Brotzen, 1934 )
|
publication ID |
https://doi.org/ 10.5252/g2010n1a1 |
|
persistent identifier |
https://treatment.plazi.org/id/03FE8790-FFDA-FFDE-68B6-F34A95100BDA |
|
treatment provided by |
Felipe |
|
scientific name |
Kujdanowiaspis podolica ( Brotzen, 1934 ) |
| status |
|
Kujdanowiaspis podolica ( Brotzen, 1934)
( Figs 5 View FIG ; 6 View FIG ; 8 View FIG ; 9 View FIG ; 11-13 View FIG View FIG View FIG ; 15-25 View FIG View FIG View FIG View FIG View FIG View FIG View FIG ; 27 View FIG ; 28 View FIG )
Phlyctaenaspis podolica Brotzen, 1934: 114 , pl. 9 figs 8-11.
Phlyctaenaspis extensa Brotzen, 1934: 116 , pl. 9 figs 4, 5.
Phlyctaenaspis rectiformis Brotzen, 1934: 117 , pl. 9 fig. 7.
Acanthaspis prominens Brotzen, 1934: 119 , pl. 9 figs 12-14.
Acanthaspis angusta Brotzen, 1934: 121 , pl. 9 fig. 16. Acanthaspis vomeriformis Brotzen, 1934: 120 , pl. 9 fig. 15.
TYPE MATERIAL. — Lectotype MB 282 (left anterolateral plate, external side, Fig. 20B View FIG ; Brotzen 1934: pl. 9 fig. 9) designated here; other specimens (paralectotypes) refered to Phlyctaenaspis podolica were not found ( Brotzen 1934: pl. 9 figs 8, 10, 11).
MATERIAL EXAMINED. — List given in Appendix 1.
TYPE LOCALITY. — Ivanye (Podolia, Ukraine; Brotzen 1934: 116) .
TYPE HORIZON. — Lochkovian (Early Devonian) (see Dupret & Blieck 2009).
DIAGNOSIS. — Small Kujdanowiaspis species with relatively coarse tubercles. Median dorsal plate with posterior sagittal crest. A suprasynarcual element covers the internal side of the median dorsal plate. Four or five post-median dorsal plates with scar insertion for a little spine for some.
REMARKS
The type locality is that of the lectotype of K. podolica (ICZN 1999:article 76.3).Original syntypes MB 87b and MB 88a for Acanthaspis vomeriformis Brotzen, 1934 (fragment of thoracic armour, print and counterprint) are refered here to Kujdanowiaspis ? podolica ; Brotzen 1934: pl. 9 fig. 15; MB 88a was collected in Unizh ( Brotzen 1934:120); other unidentified syntypes for P. extensa ( Brotzen 1934: pl. 9 figs 4, 5), P.rectiformis ( Brotzen 1934: pl. 9 fig. 7), A. prominens ( Brotzen 1934: pl. 9 figs 12, 13), and A. angusta ( Brotzen 1934: pl. 9 fig. 16) were not found; these specimens do not have type status for the species Kujdanowiaspis podolica ( Brotzen, 1934) .
Kujdanowiaspis podolica ( Brotzen, 1934)
Neurocranium
The ventral side of the neurocranium of Kujdanowiaspis is only known from Stensiö’s work on seriated sections ( Stensiö 1969); no specimen of Kujdanowiaspis exposes this side.
General aspect of the postethmoid ossification ( Fig. 5 View FIG ). The neurocranium of Kujdanowiaspis is a perichondrally ossified structure, a “primitive” condition in arthrodires (i.e. actinolepids, phlyctaeniids, and primitive brachythoracids such as Buchanosteus confertituberculatus (Chapman, 1916)) . It is composed of two separately ossified components: a rhinocapsular (or nasal capsule) and of a postethmoid ossification. Connection between these two elements may have been ligamentous and nasal capsules are rarely preserved, and consequently nicknaming them “losing-nose” fishes.
The dorsal side of the postethmoid ossification is lozengic and larger than the ventral side (specimen GGI 15-618, Fig. 6A View FIG ). Radiation centres of the overlying dermal plates, as well as the plates limits, appear as shallow depressions on the dorsal side of the neurocranium (specimen NHRM P 8458, Fig. 6B View FIG ). The neurovascular web, visible on the well-preserved specimens NHRM P 8458 and 2869a ( Fig. 6B, C View FIG ), is composed of different plexi surrounding the postorbital, otic and occipital regions, which are probably correlated with the overlying dermal bone development ( Goujet 1984).
The nuchal depression (f.dm, Fig. 6 View FIG ), visible from the level of the posterior postorbital process to the posterior edge of the neurocranium, is longer than that of Dicksonosteus . It corresponds to a thickening of the nuchal plate as suggested by Goujet ( Goujet 1984). The nuchal depression is anterolaterally surrounded by two angular ridges for the underlying anterior and posterior semicircular canals ( r.sca , r.scp , Fig. 6 View FIG ).
A pair of subparanuchal fossae (f.s.PaN, Fig. 6 View FIG ), flanking the posterior half of the nuchal depression, corresponds to a thickening of the overlying paranuchal plates (PaN). These fossae, below the ossification centre of these plates, are located at the same level than the supravagal process (p.sv, Fig. 6 View FIG ). They are associated with the internal course of the endolymphatic duct (d.end, Fig. 6B View FIG ), which is preserved as a long and thin oblique rod in specimen NHRM P 8458 ( Fig. 6B View FIG ). This duct opens internally within the neurocranium through the internal endolymphatic foramen (d.end.i, Fig. 6 View FIG ), and externally on the external side of the paranuchal plate through the external endolymphatic foramen (d.end.e, Fig. 6 View FIG ). The subparanuchal fossae are more anteriorly located than those of Dicksonosteus .
The impression of the central sensory groove (s.cc, Figs 5 View FIG ; 6 View FIG ) is deep and wide in its proximal (lateral) extremity, is thinner distally and is not visible at the level of the radiation centre of the central plates. As well, the impression of the main cephalic lateral sensory line is visible as a longitudinal groove visible in the otico-occipital region.
Several processes are developped from the anterior lateral side of the postethmoid ossification of the neurocranium. They are the ectethmoid process (p.ect, Fig. 6C View FIG ), the antorbital process (pao, Fig. 6C View FIG ), the supraorbital process (pso, Fig. 6C View FIG ), the anterior postorbital process (p.po.a, Fig. 6C View FIG ), the bifid posterior postorbital process (p.po.p, Fig. 6C View FIG ), the supravagal process (p.sv, Fig. 6C View FIG ) and the dorsal occipital process (p.occ, Fig. 6C View FIG ). The ethmoidal shelf (m.eth, Fig. 6C View FIG ) is slightly concave anteriorly and contacted the posteroventral part of the nasal capsule. The subnasal laminae (l.sbn, Figs 3 View FIG ; 4 View FIG ), formed by the postnasal plates, faced the anterior side of the ethmoid shelf. A deep dorsal canal, posteromesially directed, appears in the anterolateral angle of the shelf, and bears the olfactory nerve tractus. The ectethmoid processes protrude laterally from the ethmoid shelf and are posteriorly overlied by the antorbital processes. The latter are anterolaterally directed and overlie the ethmoid shelf. The grooves for the optic nerve (II, Fig. 6C View FIG ) and for the anterior cerebral vein (s.vca, Fig. 6C View FIG ) are clearly visible, as these structures run under the antorbital processes. Goujet (1984) considered the antorbital process as absent in Kujdanowiaspis and in other actinolepids, although the present author thinks it is more visible in Kujdanowiaspis than in Dicksonosteus : this is a real process and not only a dorsal cover for the optical nerve and the anterior cerebral vein.
The supraorbital process is large and overlies the orbit posteriorly to the ocular stalk (p.oc, Fig. 9A View FIG ). This process is absent in phlyctaeniids ( Goujet 1984). Stensiö (1963) considered it as a primitive condition in placoderms, although persistent in Pachyosteomorphi, Petalichthyida , Ptyctodontida, Rhenanida, Antiarchi and Coccosteomorphi from the Upper Devonian. Th is process would have been lost in “Dolichothoracida”, Acanthothoraci and in Upper Devonian coccosteomorphs (e.g., Tapinosteus Stensiö, 1963 ; Pholidosteus Jaeckel, 1907 ). This process is found in “actinolepids” ( Goujet 1984), and in buchanosteids ( Young 1979, 1981).
The anterior postorbital process is massive in dorsal view, but thin in ventral view, contrary to what can be observed in Dicksonosteus . As well as the different shape of this process between “actinolepids” and phlyctaeniids, the position of the foramen for the hyomandibular ramus of the facial nerve (VIIhm, Figs 5B View FIG ; 6C View FIG ) is also different. In actinolepids like Kujdanowiaspis , the foramen is placed on the distal end of the anterior postorbital process (in Heightingtonaspis , see NHM P 16030, Fig. 7 View FIG ); in phlyctaeniids and brachythoracids it is positioned just posteriorly to it. The posterodistal face of the anterior postorbital process shows a scar in Kujdanowiaspis (f.a.hm, Figs 5B View FIG ; 6A View FIG ), probably for the attachment of hyomandibular (epihyal) element, distally associated with the internal side of the submarginal plate, as is observed in other well known arthrodires (e.g., Dicksonosteus, Goujet 1984 ; Erikaspis, Dupret et al. 2007 ).
The posterior postorbital process begins at the level of the posterior semi-circular canal ampula (a.post, Fig. 11 View FIG ). It is large at its base, and distally divided into two branches. This pattern is found in all “dolichothoracid” neurocrania. A foramen between the two branches for a ramus of the vagus nerve (X1) has the same position in Dicksonosteus (see Goujet 1984).
The supravagal process is posterolaterally directed, and its shape is similar with its homologues in
Dicksonosteus and Lehmanosteus Goujet, 1984 (see Goujet 1984), though it is thinner in Kujdanowiaspis and Heightingtonaspis .
The dorsal occipital process is posteriorly directed and its shape is similar to that of Dicksonosteus (see Goujet 1984).
The impression of the semicircular canals is shallow. Th e mesial edge of the anterior canal ( r.sca , Figs 5A View FIG ; 6C View FIG ) is emphasized by the impression of the central sensory line groove (s.cc, Figs 5A View FIG ; 6C View FIG ).
The limits of the overlying dermal plates may be observed on the dorsal face of the neurocranium by the impression of their radiation of osseous fibers (e.g., NHRM P 4001, NHRM P 8458, Fig. 6C, D View FIG ) or to slight and shallow grooves corresponding to thickenings at plate contacts (e.g., GGI 15-618, NHRM P 8458, Fig. 6A, C View FIG ). Such observations may also be done in Heightingtonaspis anglica ( Fig. 7 View FIG ): it is noteworthy that the shape of the nuchal plate of Kujdanowiaspis is similar to that of Heightingtonaspis and is therefore not as elongated as supposed by White (1969: pl. II, fig. D; Fig. 14 View FIG ) (i.e. the contact between the two central plates is not so short).
Regional study of the neurocranium. A unique specimen (NASU 901/1, Fig. 8 View FIG ) from Nagiryani shows the dorsal side of the connected ethmoid and postethmoid part of the neurocranium and the related skull roof, including cheek plates.
The orbitotemporal region is located between the antorbital process and the anterior edge of the anterior postorbital process, in the first third of the neurocranium.
Dorsal side of the orbital region. It shows a foramen for the ophthalmic canal (superficial ophthalmic ramus of trigeminal nerve; c.opht, Figs 5A View FIG ; 6C View FIG ), located at the level of the supraorbital processes (NHRM P 2869a, Fig. 6C View FIG ). The homologous foramen in Dicksonosteus is located at the level of the anterior postorbital process ( Goujet 1984), i.e. in the postorbital region. In lateral view, the orbital cavity is low and elongated, as is the case in Dicksonosteus ( Goujet 1984; Fig. 9A View FIG ). The supraorbital process defines part of the roof of this cavity. It is anteriorly limited by the anterior part of the postethmoid part of the neurocranium. A postocular lamina (l.pto, Fig. 9A View FIG ), located in the posterior edge of the orbital area, links the base of the supraorbital process and the subocular shelf. The posterior myodome is located between the foramen for the pituitary vein anteriorly and the postocular lamina posteriorly.The
eye stalk (p.oc, Fig. 9A View FIG ) is located just posteriorly to the foramina for the anterior cerebral vein (vca, Fig. 9A View FIG ) and the optic nerve (II, Fig. 9A View FIG ). The ocular stalk is subquadrangular in shape and dorsoventrally elongated, as was pointed out for the first time by Goujet (1984). It is shorter than its homologue in Dicksonosteus and hence looks more like that of Romundina Ørvig, 1975 . The dorsal myodome (my.s, Fig. 9A View FIG ) is located dorsally to the eye stalk; the ventral myodome (my.v, Fig. 9A View FIG ) is anteroventrally located. Behind the dorsal myodome, the foramen for the patheticus nerve (IV, Fig. 9A View FIG ) is visible; the foramen for the communis oculomotoris nerve (III, Fig. 9A View FIG ) is located posteriorly to it (two foramina for this nerve are displayed in the neurocranium of Dicksonosteus ). A foramen for a ramus of the profundus nervus (V1a, Fig. 9A View FIG ) is located posteriorly to the latter.
The orbital region and its processes, myodomes and eyestalk are all smaller in Kujdanowiaspis than in Dicksonosteus .
According to Goujet (1984), the posterior myodome of Kujdanowiaspis is sufficiently deep to have contained both the obliquus superior and the rectus internis oculomotoris muscles. The large ventral myodome contained the obliquus inferior oculomotoris muscle. The posterior myodome contained the rectus externis oculomotoris muscle. Stensiö (1963; 1969) thought that the presence of the posterior myodome excluded the presence of an eye stalk, and that the rectus externis oculomotoris muscle was attached to its symetric on the ventral side of the neurocranium via the pituitary canal. This latter hypothesis has been refuted by Goujet (1984), as the pituitary canal is too small to contain both a muscle and a vein, and because of a double torsion of the muscle between the eye-ball and the subpituitary fossa ( Goujet 1984: 65, 66).
The rectus internis muscle was not located in the dorsal myodome in Dicksonosteus because of a pinch of the muscle ( Goujet 1984). In Kujdanowiaspis , this muscle was probably not located in this myodome because the depression is too small to contain three muscles: the rectus internis muscle was therefore attached to the upper part of the anterior side of the eye stalk ( Fig. 9B, C View FIG ). The rectus superior muscle was probably attached in the dorsal myodome ( Fig. 9B View FIG ) or to the dorsal side of the eye stalk ( Fig. 9C View FIG ).
The ventral side of the orbital region shows the scar insertions for the anterior superognathal plates (cr.ASG, Fig. 5B View FIG ), the parasphenoid (Psph, Figs 5B View FIG ; 15 View FIG ) covering the hypophyseal fenestra (fe.hyp) and the attachment areas for the palatoquadrate (a.pr.pq, Fig. 5B View FIG ). A shallow anteromesially directed groove for the interna carotida artera (s.c.int, Fig. 5B View FIG ) leading to a foramen (c.int, Fig. 5B View FIG ) runs between these two last structures courses.
The postocular region. It is located between the postorbital lamina and the anterior side of the anterior postorbital process, and is as long as the orbital region.
In lateral view, below the postorbital plate, the foramen for the profundus nerve (V2-3, Fig. 9A View FIG ), the very small jugularis fossa (f.ju, Fig. 9A View FIG ) and the two foramina for the anterior and medial jugular canals (c.v.ju.a and c.v.ju.m in front of and behind the anterior postorbital process, Fig. 9A View FIG ) are visible.
On the ventral side, the medial subpituitary fossa (f.sbp, Fig. 5B View FIG ) is laterally perforated by two foramina for the pituitary vein (c. v.pit , Fig. 5B View FIG ). This fossa is much wider than that of Lehmanosteus hyperboreus Goujet, 1984 .
The basal processes (p.b, Fig. 5B View FIG ), supposed typical for actinolepids, are situated laterally to the subpituitary fossa, with the foramina for the palatine ramus of the facial nerve (c.pal, Fig. 5B View FIG ) placed slightly posteriorly. These details are not shown in the studied material, but were demonstrated in Stensiö’s (1963) serial sections of Kujdanowiaspis neurocrania.
The otic region. Between the anterior and the posterior postorbital processes, it is very well vascularized. It shows the impression for the central sensory groove and for the anterior and posterior semi-circular canals, and a weak cohesion between the overlying nuchal and central plates in the symmetry plane: the connection was made as a slight overlap, and not a simple apposition ( Fig. 6 View FIG B-D).
Unfortunately, the only specimen exposing the otic region in lateral view is very badly preserved (NHM P 20 588).
Short grooves and the long lateral groove for the jugular vein are observed between the two postorbital processes. This groove is more open than that of Dicksonosteus . It is connected anteriorly to the medium foramen for the jugular vein (c.v.ju.m, Fig. 5B View FIG ), and posteriorly by the posterior foramen for the jugular vein (c.v.ju.p, Fig. 5B View FIG ).
Specimen NHM P 16030 (referred to Heightingtonaspis anglica , Fig. 10 View FIG ) shows three foramina on the anterior branch of the posterior postorbital process: one mesial for the post-trematic ramus of the glossopharyngeal nerve (IXpt), and two laterally for the branchial vein (v.br) (as these two foramina are very close to each other, the vein was maybe bifid). Homologous structures were observed by Stensiö (1963) in Kujdanowiaspis , using the seriated sections method.
A foramen is observed slightly anteriorly to the posterior postorbital process in Heightingtonaspis anglica (on the ventrolateral edge of the neurocranium). This may correspond to the exit of the glossopharyngeal nerve (IX) or its pharyngeal ramus (IXph). Because of its big size, the “glossopharyngeal hypothesis” is preferred.
The occipital region. It is located behind the posterior postorbital process.
Some foramina for the vagus nerve (X), firstly identified by Stensiö (1963), are visible on the lateral side of the occipital region, between the posterior postorbital and supravagal processes.
Foramina for the craniospinal nerves occur between supravagal and occipital processes (spioa,b,c, Figs 5B View FIG ; 6A View FIG ).
The ventral side of the occipital region in Heightingtonaspis anglica shows the chordal ridge (r.ch, Fig. 10 View FIG ), which becomes thinner anteriorly. The posterior side shows the chordal fissure (f.ch, Fig. 10 View FIG ), posterolaterally to which lay the – non preserved – occipital facets that were articulated to the synarcual. The groove for the laterodorsal aorta (s.ald, Fig. 10 View FIG ) is located ventrally to the occipital facets, is anteriorly directed and separated from the craniospinal foramina by a slight crest. This groove bifurcates at the level of the internal endolymphatic foramen. One ramus surrounds the semi circular canals and the saccula (groove for the anterior arterial root, s.ra, Fig. 10 View FIG ). The other ramus is straight and anterolaterally directed until the posterior postorbital process (groove for the branchial aorta, s.a.br, Fig. 10 View FIG ).
Central nervous system
The central nervous system was reconstructed by Stensiö (1963) using seriate sections, but photos of a mechanically prepared specimen (NHRM P 8466a, Fig. 11 View FIG ) were never published. Only the mesencephalic and the anterior rhombencephalic parts of the neurocranium are visible in dorsal view.
There is no constriction between the mesencephalon and the rhombencephalon. The pathetic nerve only (IV, Fig. 11 View FIG ) is visible; the communalis motor ocularis nerve (III) is located on the ventral side, and hence invisible here.
The anterior part of the rhombencephalon corresponds to the trigeminal nerve recessus (V). This subdivision corresponds to three quarters of the encephalon, as is the case in Dicksonosteus . The anterior region is located between the trigeminal nerve recessus and the facial (VII, Fig. 11 View FIG ) and acoustic (IX) nerves recessus. In Dicksonosteus , the facial and acoustic nerves branch independently from the metencephalon. The posterior part of the rhombencephalon is located posteriorly to the acoustic canal, and contains the medulla oblongata.
The proportions and the positions of the different encephalic structures reveal a primitive cerebral condition, because this pattern is presently found in extant gnathostomes embryos: a very anterior position of the hypophysis (Moy- Thomas & Miles 1971), a nerves seriation, the same diameter between the encephalon and the spinal chord, and a very elongate myencephalic region (compared to the rest of the brain) are present in the first stages of the formation of the encephalon of extant vertebrates. Hence, the pattern of the placoderm brain could represent that of the ancestral gnathostome brain pattern.
Dermal bones of the skull
The plates of the skull roof are preferentially compared with those of two other actinolepids: Heightingtonaspis White, 1969 and Eskimaspis Dineley & Liu, 1984 .
General shape ( Fig. 12 View FIG ). The skull roof of Kujdanowiaspis is slightly wider than long. Its minimal width is located between the orbital notches, and its maximal width between the postmarginal plates, as is the case in all “actinolepids”. It is covered by tiny rounded tubercles (about 1 mm in diametre), with a roughly concentric distribution around the radiation centre of each plate.
On the internal side, the plates limits are indicated by very slight ridges, also impressed on the dorsal side of the neurocranium (see above). It is noteworthy that the plate sutures on the external surface do not strictly match with internal sutures, because of some slight overlappings.
The tuberculated layer of the specimen GGI 15-610 ( Fig. 13A View FIG ) was partly destroyed in order to reveal growth stages (s.Tn, Fig. 13A View FIG ): some successive radiating osseous fibres, perpendicular to the plate limits, are also visible.
The rostrum. The rostrum is rarely preserved (only one specimen), as the two neurocranial components (ethmoid and postethmoid) were weakly attached. Nevertheless, the specimen discovered by V. Voichyshyn (NASU BP 901/1) provides some informations. It is also very similar to the one described (and attributed to K. buczacziensis ) by Stensiö (1942).
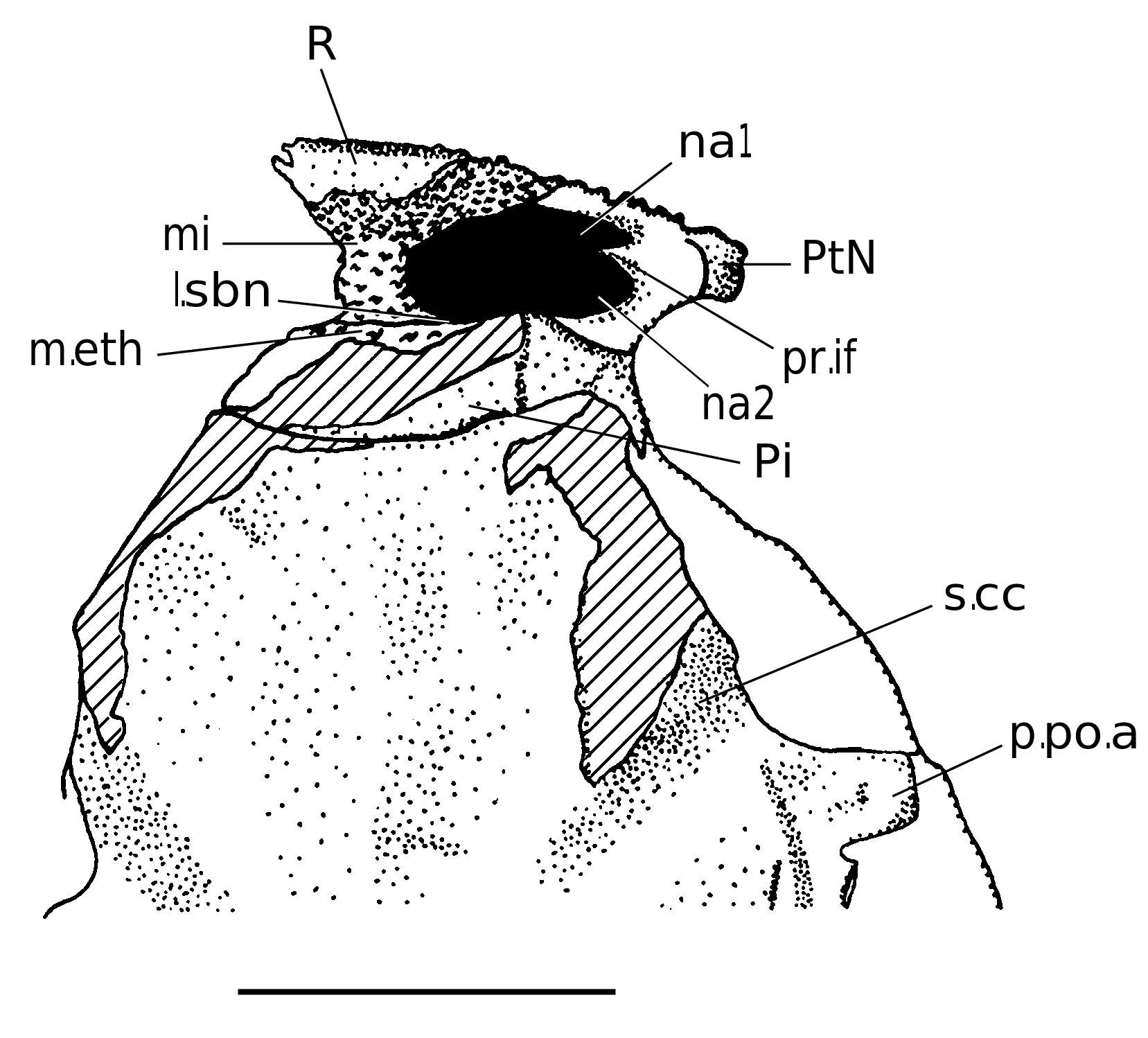
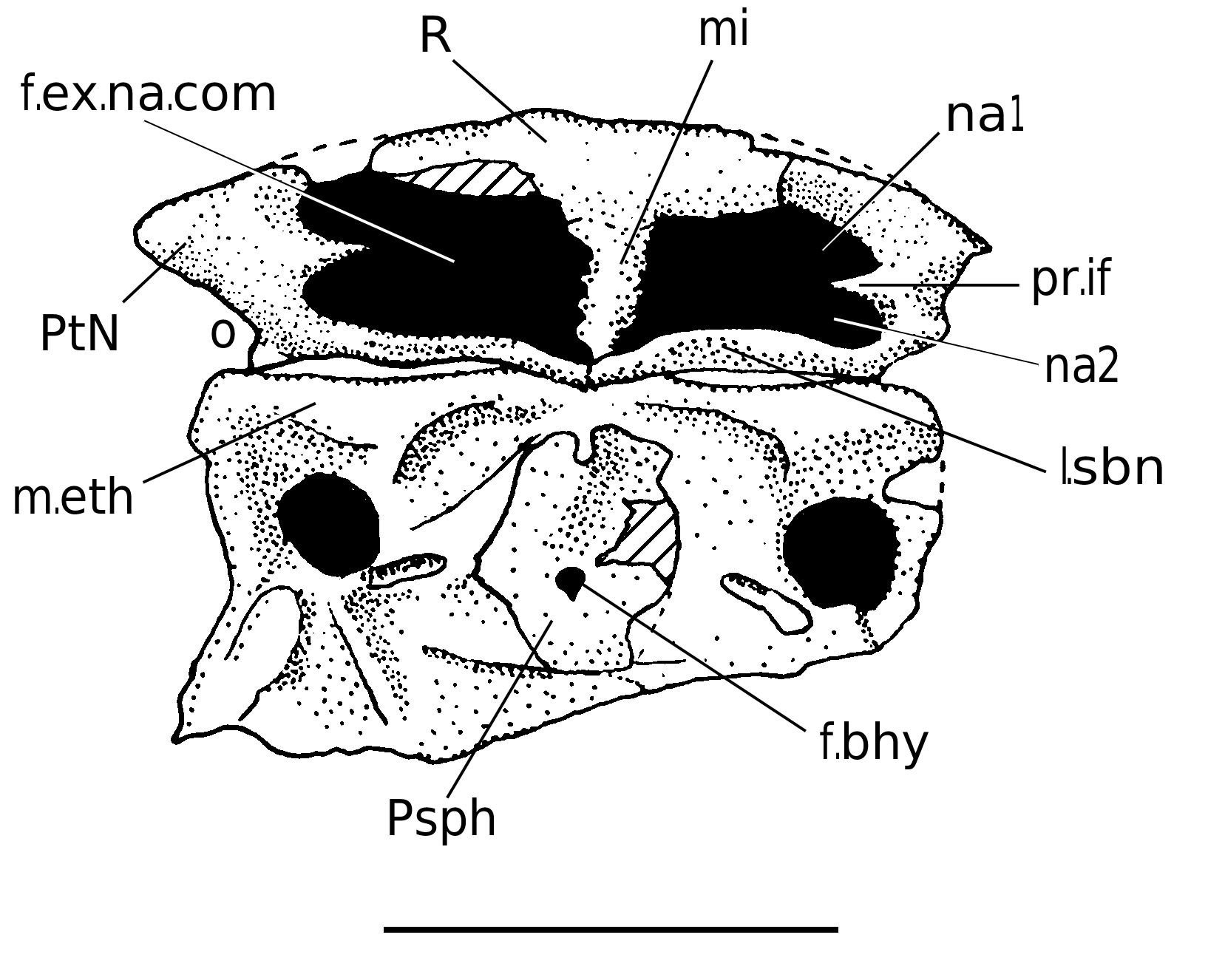
Dorsally, the rostral capsule is convex, wide and short, which is a primitive arthrodire feature among arthrodires ( Goujet 1984). In internal view, the posterior half exposes a deep depression for the pineal pit (f.Pi, Fig. 8 View FIG ). Neither radiation centres nor plates limits are visible on this specimen. Neverthless, it is possible to suppose that the limit betwen the rostral and pineal plates may correspond to the groove delimiting anteriorly the anterior edge of the pineal pit.
The connection between the ethmoid and postethmoid part of the skull roof is vertical and inflexes at the level of the ethmoid shelf (specimen NMH P 18277, Fig. 9 View FIG ).
The rostral plate (R). Its radiation centre is probably located on the anterior side. Dorsally, the plate is slightly trapezoidally shaped, and is surrounded laterally by the postnasal plates and posteriorly by the pineal plate.
The pineal plate (Pi). It shows slightly convex anterior and posterior edges. Its radiation centre is anterior to the “pineal pit”. This last structure does not correspond to a real foramen: the bone layer was very thin at this place and therefore easily breakable (see Stensiö 1942: 5, caption of fig. 1).
The postnasal plates (PtN). They are the lateral components of the dermal nasal capsule. The sutures with the rostral and the pineal plates are not visible, nor are the radiation centres (which would be at the level of their lateral extremity). Contrary to what Stensiö figured (1942: fig. 2), the supraorbital groove does not run behind the posterior edge of the postnasal plates, but in the most lateral part of the posterior edge of the pineal plate. Nevertheless, preorbital plates certainly contacted the postnasals.
The postnasal plates are small elements in Kujdanowiaspis podolica , compared with those of Bryantolepis brachycephala , where the anterior edge of the preorbital plates corresponds to the suture of the postnasals (see Denison 1958: fig. 105C), whereas in Actinolepis magna Mark- Kurik, 1973, the postnasals contact both preorbital and postorbital plates.
The eye opening with a sclerotic ring of the specimen from Nagiryani NASU 901/1 (scl, Figs 8 View FIG ; 12 View FIG ) is preserved and shows a clear (though short) contact between the postnasals and preorbital plates.
The preorbital plates (PrO). They are paired, small and pentagonal. Their anterior edge is slightly concave and receives the posterior part of the pineal plate. Their radiation centre corresponds to both the geometrical centre and the posterior end of the supraorbital groove. Their posterior edges are perpendicular to the symmetry plane but are never confluent with each other (i.e. the left preorbital contacts the two central plates). The limit between these plates is visible on specimen NHRM P 8420 ( Fig. 13B View FIG ). Each plate shows the anteroposteriorly and slightly mesially directed supraorbital groove (soc), which forms a ridge on the ventral side. A few specimens show a profundus sensory line (pfc, e.g., NHM P 20773, Fig. 13C View FIG ). Laterally, the preorbital and postorbital plates show a shallow orbital notch.
The postorbital plates (PtO). They are slightly larger than the preorbitals, and smaller than the centrals. The radiation centre is situated at the level of the trifurcation between the central and the two rami of the infraorbital sensory lines. The posterior
PM
edge of the plates is displaced across the infraorbital groove which divides the plate into a dorsal (mesial) and a lateral laminae, which is very different from Denison’s reconstruction (1958: fig. 105F). The most posterior edge, located on the dorsal lamina, contacts the anterior “process” of the paranuchal plate and laterally forms a small “canopy” over the infraorbital groove. The lateral lamina contacts the marginal plate. The ventral side of the plate bears ridges which correspond to the external infraorbital and central grooves.
The central plates (C). They cover the otic region of the neurocranium. They are slightly larger than the postorbitals. The radiation centre, at the geometric centre of the plate, is located around the extremities of the central groove and of the middle and posterior pit-lines.
The marginal plates (M). They are smaller than the preorbitals. The radiation centre does not correspond to the geometric centre but is located in the mesial edge of the plate, slightly anteriorly to the underlying posterior postorbital process. The plate does not extend mesially from the infraorbital and the main sensory grooves, contrary to what Stensiö assessed (e.g., Stensiö 1945: figs 8A, 9A, 11B; 1963; 1969, see also Denison 1958: fig.105F; 1978: fig. 31B). The postmarginal groove runs all along the plate in Kujdanowiaspis podolica , but stops before the lateral edge in Eskimaspis .
The postmarginal plates (PM). They are slightly smaller than the marginals and are subquadrangular ( Fig. 13C View FIG ). They contact anteromesially the marginals, and posteromesially the paranuchals. The radiation centres correspond to the extremity of the postmarginal groove. A size gradient in the tuberculation indicates that these plates were probably somewhat covered by the “opercular” submarginal plate (SM), with the most lateral tubercles being much finer than the others (see specimen NHM P 20773, Fig. 13C View FIG ).
The nuchal plate (N). It is long, as is the case in most actinolepids (except in Actinolepis , Bryantolepis , Eskimaspis , and Simblaspis where it is
relatively short). Its anterior part is lanceolate and separates the posterior half of the central plates ( Fig. 13D View FIG ). The radiation centre is located at the geometric centre. Its maximal width at the level of the contact with the central and paranuchal plates, then it slightly decreases posteriorly. The nuchal plate thickens from the radiation centre toward the posterior edge, and shows a shallow inverted “V”-shaped hump. The posterior edge is smooth; this may be related to the presence of some extrascapular element(s), not encountered anyway in the material. One specimen (NHRM P 8426, Fig. 13E View FIG ) is an isolated nuchal plate in dorsal view, exposing the insertion surface for the centrals. The lateral edges of the nuchal cover the mesial border of the paranuchals.
The paranuchal plates (PaN). They are very wide and roughly heptagonal. The radiation centre corresponds roughly to the geometric centre. The groove for the main sensory line demarcates a dorsal and a lateral laminae. The dorsal lamina shows a slender anterior process which contacts the posterior edge of the postorbital plate, as is the case in, for example, Heightingtonaspis and Eskimaspis . The combination of these two processes entirely separates the central and marginal plates. The centre of the paranuchal plate is thickened. The external foramen for the endolymphatic duct (d.end.e, Figs 12 View FIG ; 13B, C View FIG ), the posterior end of the posterior pit-line (pp, Fig. 13B, C View FIG ) and the occipital cross-commissure (occ, Fig. 13B, C View FIG ) are visible around a flexure of the main sensory groove. The dorsal lamina forms a little “roof” – continuing that of the postorbital plate – over the sensory groove. The tuberculation is uniform on the plate and does not show any overlapping by the submarginal plate.
Some specimens show tubercles of different sizes. But there is no evidence to determine rather if the smallest or the largest are of second generation. Indeed, second generation tubercles used to cover the first generation which was then resorbed. Have the smallest tubercles, between the larger ones, not been covered, or did they appear during the second generation? It is nevertheless noteworthy that some Arthrodira show in cross sections of the exoskeletal plates a number of tubercle generations following
one another (e.g., Homosteus Asmuss, 1856 , Luetkeichthys Bystrow, 1957 ). In other cases resorption of earlier generations took place.
The main sensory line groove shows two flexures on the paranuchal plate: an anterior one with a wide mesial concavity, and a posterior one with a sharper laterally directed concavity. Mesially to this last flexure are positionned the posterior pitline extremity, the external foramen for the endo- lymphatic duct and the lateral end of the occipital cross commissure. The groove for the main sensory line runs backwards until the posterolateral corner of the plate.
Dermal elements of the ventral side
The dermal elements of the ventral side of the neurocranium and of the palate are rarely and poorly preserved.
The anterior superognathal plates (ASG). They are paired, and located on the lateral half of the ventral side of the ethmoid shelf. These plates are not entirely preserved, but their shape can be deduced from their scar insertion (wider than long) on the ventral side of the neurocranium ( Fig. 5B View FIG ). There is no evidence concerning the ossification centre of these plates in Kujdanowiaspis .
The specimen NHM P 18277 ( Fig. 9A View FIG ; Goujet 1984: pl. 29 fig. 1) shows the anterior and the left lateral sides of the anterior superognathals. Each plate may be divided in two parts: a flat osseous basal lamina (connecting the ethmoid shelf; b.ASG, Fig. 9A View FIG ), and a tuberculated apical part. The anterior edge of the plate shows six tubercles, plus two others slightly posteriorly.
This kind of plate is primitive compared to the shearing ones of the ptyctodonts (e.g., Ctenurella Ørvig, 1960 , see Long 1997) or the biting ones of
some derived brachythoracids (e.g., Dunkleosteus Lehman, 1956 ). The posterior superognathals are not known. It is supposed that they were attached to the autopalatine (also unknown), presumably connected to the ventral side of the neurocranium, as it occurs in the phlyctaeniid Dicksonosteus ( Goujet 1984) and other arthrodires.
Parasphenoid (Psph). Stensiö (1942: figs 4A, B, 5) first figured the parasphenoid (Psph) of Kujdanowiaspis buczacziensis , and considered it as a “tooth-bearing thickening on the lower side of the neurocranium presumably representing the fused anterior superognathals of both sides in Brachythoracid Euarthrodires”. He figured it as a very anteroposteriorly elongated hexagon. He only considered this element as a true parasphenoid in 1945 (addendum, p. 67), and reconstructed it a bit wider than before, but with uncertain edges ( Stensiö 1963).
Only one photo made under UV light of the ventral side of a neurocranium (specimen NHM P 20542a, Fig. 15 View FIG ; this photo is the only material the author accessed to) shows an almost complete parasphenoid (only the lateralmost flanges seem to be lacking), with the surrounding ventral side of the neurocranium which size is that of K. podolica . It is quite quadrangular in shape (33 mm wide) and is located anteriorly to the subpituitary fossa. The central part of the element is clearly visible owing to its thick spongiose bone stucture. The anterior and lateral edges are concave and the posterior edge convex. The left lateral edge shows a tiny notch. The thin layer of bone at the centre of the parasphenoid is broken, probably during preparation. Hence, as noticed by Stensiö (1942), the hypophyseal fenestra was probably ventrally closed.
It is impossible to determine whether K. podolica did really possess a parasphenoid of different shape than that of K. buczacziensis , because of the scarcity (one in each species) and the preservation of the material.
Dermal elements of the cheek
The dermal elements of the cheek are known by one specimen, exposing the external side only (NASU 901/1, Fig. 8 View FIG ), which size and ornamentation belong clearly to K. podolica . The author only studied a photo of the specimen, nicely communicated by V. Voichyshyn (NASU).
Suborbital (SO) and the postsuborbital plates (PSO). They show the typical tuberculated ornamentation. The anteriormost part of the suborbital plate corresponds to a ventral slender lamina that borders the ventral edge of the orbit (suborbital lamina, l.so, Fig. 12 View FIG ), and contacts sclerotic elements of the eye. The orbital notch is large and deep. The posterior part of the suborbital plate is higher. In its anterior part, the infraorbital groove (ioc) is vertical and surrounds the orbit in the suborbital lamina. The supraoral groove is parallel to the ventral edge of the suborbital plate, and seems to contact anteriorly the infraorbital groove (sorc) at the level of the radiation centre. This supraoral groove is larger than that of Dicksonosteus , in which it does not contact the infraorbital groove, but Goujet (1984) proposed than the connection may have occured in the most superficial layers of the dermis.
Though the internal side is unknown, the plate was probably connected to the palatoquadrate, itself linked to the neurocranium.
In its posteriormost part, the suborbital plate seems to overlap the postorbital plate (PSO, Figs 8 View FIG ; 12 View FIG ), which is much higher than long.
Submarginal plate (SM). It is the most posterior element of the cheek and assumed the opercular function in placoderms. The anterodorsal extremity shows a little spur which overhang the dorsal edge of the suborbital and the postsuborbital plates. Because of the tuberculation zonation observed on the postmarginal plate ( Fig. 13C View FIG ), the submarginal plate certainly covered the most lateral part of the postmarginal.
Epihyal element (l.ext.Hm, Fig. 8 View FIG ). It was probably cartilaginous, and hence was not preserved. But both its shape and position can be determined owing to the print let on the external side of the submarginal plate (also occurs in Erikapis zychi ; see Dupret et al. 2007). An oblique shallow and slender broken rodlike structure, with osseous fibers oriented along the great axis, is visible posteriorly to the anterodorsal spur of the submarginal plate. This rod would be the external expression of the epihyal element, fused to the internal side of the submarginal plate, as is the case in Sigaspis lepidophora Goujet, 1973 and Dicksonosteus arcticus Goujet, 1975 . The disposition of this rod is similar to the ones assessed in the two previous cited species.
The thoracic armour ( Figs 16-25 View FIG View FIG View FIG View FIG View FIG View FIG )
General features. The dermal skeleton is relevant to discriminate the two species Kujdanowiaspis podolica and K. buczacziensis . The available material comprises various incomplete shields, showing connected plates and many isolated plates showing overlap areas, both in external or internal views.
The thoracic armour covers the body part comprised between the bottom of the branchial cavity and the anus. As in Sigaspis lepidophora Goujet, 1973 , the anus was probably located just behind the posteroventral plates.
This armour is composed of all the classical plates previously described in arthrodires. No plate is regressed nor lacking as it occurs in some Brachy- thoraci (e.g., Aspinothoraci, Pachyosteomorphi), Phlyctaenioidei (lacking anteroventral plates) or Phyllolepida (lacking anterior and/or posterior median ventral plates). On the contrary, actinolepids were supposed unique among arthrodires because of the possession of some anteroventral plates (see below).
The dorsal side of the armour is very high, convex and “roof-shaped”. The plastron is slightly convex posteriorly (at the level of the “boxshaped” posterior ventrolateral plates, flexing dorsally to meet the posterolaterals). No trace of annular thickening (“bourrelet annulaire” of Goujet 1984), as known in Dicksonosteus or Arctolepis , is visible.
Extrascapular plates (ESC, Fig. 16D View FIG ). They are unknown, but the smooth areas on the posterior margin of the nuchal plate and on the anteromedial edge of the median dorsal plate indicate that these elements did exist, either as a pair of plates
or just a single element. No extrascapular plate was refered to Eskimaspis and Heightingtonaspis ( White 1961, 1969; Dineley & Liu 1984).
Median dorsal plate (MD). It is almost as wide as long (previously considered as a characteristic of the actinolepids), whereas it is much longer than wide in phlyctaeniids like Dicksonosteus ( Goujet 1984) . The median dorsal plate of Kujdanowiaspis is roughly pentagonal, high and roof-shaped, with a rounded extremity pointing posteriorly. The tubercles are concentric around the radiation centre located one third of the length from the anterior margin. A posterior sagital crest a few millimeters high extends posteriorly from the radiation centre (cr.pd, Figs 16A, C, D View FIG ; 17 View FIG ; 18 View FIG A-E).
Two small anteromesial notches probably housed the extrascapular element(s) (sr. ESC, Fig. 17A View FIG ). These notches are more pronounced in Eskimaspis heintzi ( Dineley & Liu 1984: figs 1A, 5A).
A few specimens (GGI 15-612 and NHM P 20616, Fig. 18C, D View FIG ) show a strong tubercle differentiation in size, with bigger tubercles at the periphery, related to the age of individuals.
The internal view exposes well the overlap areas for the anterior and posterior dorsolateral plates ( Figs 17B View FIG ; 18E, F View FIG ).
As in other “Dolichothoraci”, there is no median keel, but just a small medial groove (ra, Figs 17B View FIG ; 18F View FIG ). This groove corresponds to a low and quite wide crest, that would be an attachment area for muscles connected to the synarcual and the vertebral column, as it occured in Dicksonosteus (see Goujet 1984; and below).
The median dorsal plate of Heightingtonaspis anglica is unknown. That of Eskimaspis shows two unornamented areas on the anterior edge, probably for overlap by extrascapular elements (not found) ( Dineley & Liu 1984: figs 1A, 5A).
Anterior dorsolateral plate (ADL, Figs 16 View FIG ; 19 View FIG ). It is a flat, anteroposteriorly lengthened plate. It is noteworthy that for phlyctaeniids like Dicksonosteus , Arctaspis , Arctolepis and Heintzosteus , the plate
shows a flexure dorsally to the main sensory line groove. The lack of this flexure in Kujdanowiaspis is compensated by the highly arched median dorsal plate.
The anterior part of the anterior dorsolateral shows a long and thin dorsal process (p.d) extending anterolaterally to the median dorsal. A thin ventrolateral process covers the anterior part of the obstantic lamina of the anterolateral plate (p.obst).
A smooth articular lamina underlied the paranuchal plate on the anterior margin of the plate. This “sliding neck joint articulation” is typical for actinolepids ( Miles 1973) and phyllolepids ( Long 1984). The dorsomesial part of this articular lamina is larger than the ventrolateral one.
The anterior dorsolateral plate is broadly overlapped by the median dorsal plate anterodorsally and by the anterolateral plate ventrolaterally (cf. specimens NHRM P 8432, Fig. 19B View FIG ), and overlaps the posterior dorsolateral plate posteriorly.
The posterior edge of the plate is approximately located at the level of the posterolateral flexure of the median dorsal plate.
The tubercles form rows parallel to the main sensory line groove.
Specimen NHRM P 8432 ( Fig. 19B View FIG ) shows a single row of tubercles within the main sensory line groove, suggesting a double line, as is known
in extant fishes, or in the brachythoracid Holonema Newberry, 1889 (see for example Lelièvre et al. 1990).
A tiny dorsal branch of the main sensory line groove at the anteriormost part of the plate is visible: “accessory groove” (l.acc) (see specimens NHRM P 8419, NHRM P 8432, GGI 15-615, NHM P 20616, NASU 28555, Figs 16A, C, D View FIG ; 19B View FIG ). This groove can also be observed in phlyctaeniids like Dicksonosteus arcticus , Arctolepis decipiens Woodward, 1891 , or Heintzosteus brevis ( Heintz 1929b; Goujet 1984), and is much longer in some (e.g., Holonema westolli Miles, 1971 ; dlc, in Denison 1978). This character is too much frequent to be considered as a simple teratologic feature.
Posterior dorsolateral plate (PDL, Figs 16 View FIG ; 18C View FIG ). It is quadrangular and twice shorter than the anterior dorsolateral. The groove for the main sensory line runs until the geometric centre that corresponds to the radiation centre. Tubercles are arranged as parallel rows along the groove.
The posterior dorsolateral plate is overlapped by the anterior dorsolateral plate anteriorly and by the median dorsal plate dorsally; it overlaps the
anterolateral plate ventrolaterally, and at a lesser degree the posterolateral plate.
The posterior dorsolateral plate of Kujdanowiaspis podolica is higher than that of Eskimaspis in which the groove for the lateral line is visible all along the plate.
Anterolateral plate(AL, Fig. 20 View FIG A-D).It is trapezoidal. The radiation centre is located slightly ventrally to the
geometric centre (e.g., specimen MB 282; Brotzen 1934: fig. 9; Fig. 20B View FIG ). From this radiation centre, four crests reach the angles of the plate, hence defining four triangles:the anterior (bearing the postbranchial and obstantic laminae, l.pbr and l.obst, Fig. 20A, B View FIG ), dorsal, posterior and ventral. The tubercles are concentrically arranged around the radiation centre. The tubercles of the postbranchial lamina are finer than elsewhere on the plate. This was covered by the back of the skull roof and the submarginal plate. The postbranchial lamina is oriented at almost 90° from the rest of the anterolateral plate.
The pectoral notch (e.pec, Fig. 20 View FIG A-D) is a two dimensioned structure emerging the posterior edge of the anterolateral plate (compared to the pectoral fenestra which is a three dimensioned structure), and is shallower than that of Dicksonosteus ( Goujet 1984) . The suprapectoral lamina is high (almost one half of the height of the plate; e.g., specimen NHM P 18241; Fig. 20C View FIG ).
The contact faces for adjacent plates are visible on the internal side of specimen NHM P 18241, owing to the radiating stries( Fig.20C View FIG ): that for the posterolateral plate being smallest (s.r.ADL, s.r.PDL, s.r.PL, Fig.20C, D View FIG ). The area for the spinal plate is very low (s.ins.Sp, Fig.20C, D View FIG ) and inserted in a groove of the interolateral and spinal plates (s.ins.AL, Fig. 21B View FIG ).
In Heightingtonaspis anglica and Eskimaspis heintzi , the pectoral notch is shallower than in K. podolica . Nevertheless, the anterolateral plate of Heightingtonaspis anglica is of higher proportions than those of K. podolica and Eskimaspis heintzi .
Posterolateral plate (PL). It is illustrated by one isolated plate (specimen Pi 1202, Fig. 20E View FIG ). The shorter (anterior) overlap area connected the anterolateral plate, as the larger (ventral) one was overlapped by the posterior ventrolateral plate. A longitudinal ridge, or lateral crest, separates the posterolateral plate in two laminae, in the middle of which is located the radiation centre. As is the case in Dicksonosteus , the ventral part is the smallest, and shows one single row of tubercles decreasing in size posteriorly. The dorsal part shows two sizes of tubercles: some very fine around the radiation centre, and some larger at the peripheries beyond. The internal side is not exposed.
Interolateral plate (IL, Figs 16 View FIG ; 21 View FIG ; 22). It is transverse, elongated, articulating with its antimere in the midline, but its sutures are rarely visible ( Fig. 21 View FIG ), and no isolated plate is known. As in all arthrodires ( Denison 1958; Stensiö 1959), the interolateral plate consists in two laminae: a ventral one (external) forming the anterior edge of the ventral armour, and an ascending one (internal) which is laterally associated with the postbranchial lamina of the anterolateral plate in order to close posteriorly the branchial cavity.
The ascending lamina widens laterally and its tuberculation is always very fine.The interolateral plates of Eskimaspis and Heightingtonaspis are straighter than that of K. podolica . Moreover, the dorsal lamina of Eskimaspis heintzi is a little bit higher and unornamented ( Dineley & Liu 1984).
The tubercles form parallel rows on the ventral lamina, the biggest being on its anterior side.
A shallow groove (s.av, anteroventral sulcus) is visible on the ventral external side, and is oriented in the plate direction before turning (at almost 90°) on the spinal plate where it is shallower (cf. NHM P 34 852 and NHM P 18 140, Fig. 21A, C View FIG ). Miles (1965) considered this sulcus functionned as a probable locus for neuromasts, or for Lorenzini ampullae, or both. As this sulcus is tuberculated, Goujet (1984) does not allow any peculiar function to it.
Paired anteroventral plates (AV). They are triangular and probably anamestic (“filling in” plates). They articulate with the interolateral and anterior ventrolateral plates (specimen GGI 15-634, Fig. 22A). The tuberculation is very fine. The radiation centre corresponds to the geometric centre.
Concerning the “evolution” of these anteroventral plates among placoderms,some authors (e.g., Stensiö 1971; Ørvig 1975) considered that they were lost by fusion with an adjacent plate in phlyctaenioids (i.e. phlyctaeniids and brachythoracids). The anteroventral plate is found only in actinolepids, some petalychthyids (e.g., Eurycaraspis incilis Liu, 1991 ), and acanthothoracids (e.g., Romundina Ørvig, 1975 )and has been recently described in the groenlandaspidid Mulgaspis Ritchie, 2004 . But as noticed by Goujet (1984), if fusion occured, one would have found some teratologic specimens. But it is noteworthy that this
last argument is far from receivable, because of the many biases occuring during fossilization. A better knowledge of the transition between actinolepids and phlyctaenioids is required.
Anyway, as these anteroventral plates are no more unique in actinolepids, they cannot be diagniostic of the group. Otherwise, alone they cannot provide a monophyletic status to actinolepids.
Anterior ventrolateral plates (AVL). Their radiation centre corresponds to the geometric centre (if abstraction of the pectoral sinus, i.e. the pectoral notch). As is the case in most “dolichothoracids”, this plate is not flat (see specimens RAS 2172-2,
Fig. 22C, and NHRM P 8512, Fig. 21B View FIG ). A mesioventral and a lateral laminae are separated by a ventrolateral crest which is more marked posteriorly. The lateral lamina is divided in two parts: a long infraspinal lamina (limited by the suture with the spinal plate anterolaterally, and by a posterolateral tip posteriorly), and a lateral lamina (edging the pectoral fenestra ventrally), and developing a postpectoral lamina. This latter lamina, associated with that of the posterior ventrolateral plate, connects with the anterolateral plate.
The anterior and lateral edges of the anterior ventrolateral plate are straight, but the mesial and posterior edges are rounded and overlap the adjacent
plates (anterior and posterior median ventral and posterior ventrolateral plate).
The tuberculation shows a slight regional differentiation with finnest tubercles on the ventral surface, and some slightly coarser tubercles on the lateral laminae.
The margins of the scapulocoracoid attachment are visible internally.It is associated with tiny foramina, and sometimes traces for neurovascularization of this area (c.cut.v, Fig. 22A).
In Kujdanowiaspis podolica and Eskimaspis , the lateral edges of the anterior ventrolateral plate are less angular than in Heightingtonaspis .
The posterior ventrolateral plate (PVL). It is as long as the anterior ventrolateral plate (but shorter than that of Eskimaspis ). Contrary to other paired plates of the armour, it is not strictly symmetric with its antimere, with the left plate slightly overlaping ventrally (externally) the right one. The suture between these paired elements is therefore rather simple, as in other
actinolepids (e.g., Sigaspis lepidophora Goujet, 1973 ). In phlyctaeniids like Arctolepis, Discksonosteus or Heintzosteus , this suture shows a sinusoidal pattern ( Goujet 1984). The posterior ventrolateral plate is overlapped anteriorly by the anterior ventrolateral plate, and mesially the posterior median ventral plate.
The plates of the plastron are partly disassembled on NASU 25567a ( Fig. 24A View FIG ), to reveal the smooth overlap areas and the two laminae of the posterior ventrolateral plates, the lateral lamina connecting the anterolateral plate.
The posterior ventrolateral plate is composed by two laminae: a folded ventromesial and a lateral, visible on specimen NASU 25567a ( Fig. 24A View FIG ), the latter connecting the anterior posterolateral plate.
A regional differentiation in the ornamentation is visible on the specimens GGI 15-624 and GGI 15- 625 ( Fig. 22D, E). As for the anterior ventrolateral plate, the lateral lamina shows bigger tubercles than the ventral. The radiation centre is slightly anteriorly displaced from the geometric centre. The lateral lamina is much shorter than the ventral one.
There is no “annular thickening” on the internal side as it occurs in Dicksonosteus ( Goujet 1984) .
Anterior median ventral plate (AMV). It is short and wide, pentagonal and probably anamestic ( Fig. 23A View FIG ). It is slightly pointing anteriorly. The radiation centre corresponds to the geometric centre. It is overlapped anterolaterally by the anterior ventrolaterals. It overlaps posteriorly the posterior median ventral plate. Tubercles are fine.
Posterior median ventral plate (PMV, Fig. 23B, C View FIG ). It is almost twice longer than wide, and also probably anamestic. The radiation centre is slightly posteriorly located compared to the geometric centre. The ornamentation is slightly coarser posteriorly. The plate is overlapped by the anterior median ventral plate anteriorly, by the anterior ventrolateral plates laterally, and by the posterior ventrolateral plates posterolaterally.
The specimen NHRM P 8440 ( Fig. 23B View FIG ) shows in its posterior part a large notch which could be interpreted as 1) a simple corrosion gap (but it is curiously symmetric), 2) a non-ossification during
life of the animal, or 3) an insertion area for a surnumeral plate (but no overlapping area is visible, at least on this side).
Spinal plate (Sp). It is long (see NHM P 34852, Fig. 21A View FIG ) and terminates as a smooth tip, slightly
anteriorly to the level of the posterior edge of the posterior ventrolateral plates (see specimens NHRM P 8512, NASU 25567a, Figs 21B View FIG ; 24A View FIG ). The radiation centre corresponds to the tip apex. The plate is situated in a slightly upper plane than the ventral armour surface ( Fig. 16D View FIG ). As in all arthrodires, it was filled in with cartilage (prolongation of the scapulocoracoid, see below).
The lateral sides are ornamented with rough tubercles longitudinally disposed (about two rows). The dorsal and ventral sides are covered with thin tubercles. The trench in which the anterolateral plate articulated is visible on the specimen NHRM P 8512 ( Fig. 21B View FIG ). When well preserved, the free mesial side of the spinal plate shows slightly anteriorly pointed spur-like denticles (5 on NASU 25567a, Fig. 24A View FIG ). Because of the preservation of the material, no variation in the number of denticles can be provided.
The anteroventral sulcus continues on the ventral side of the spinal plate, its height decreasing distally. Another groove runs along the mesial edge of the ventral side, and a third one on the mesial side of the plate.
The spinal plate articulates with the anterolateral plate dorsally, with the interolateral plate anteromesially, and with the anterior ventrolateral plate mesially (along the infraspinal lamina of the latter).
Dermal elements of the tail
The plates covering the tail are found in association (subarticulated and slightly displaced) with the body armour in two specimens (NHM P 18140 and NASU 25567c; Figs 21C View FIG ; 24A View FIG ); they are more numerous as isolated specimens (see Appendix 1; Fig. 24 View FIG B-H). Scales are only preserved on specimen NASU 25567c. Two isolated postmedian ventral plates have been identified.
Postmedian dorsal plates (PMD, Figs 21C View FIG ; 24 View FIG A-I). They are small finely tuberculated elements covering the dorsal side of the tail. The first element was probably weakly inserted under the median dorsal plate (as no overlap area or scar is visible on the internal side of the median dorsal plate). Contrary to Dupret (2004: fig. 5), the first postmedian dorsal plate does not contact the posterior dorsolateral plate, because 1) tubercles are all of the same size on the tail dermal elements, 2) the insertion surface is always medial and does not extend very much laterally, and 3) the plates are not high enough (even if diagenesis plays some role in it).
Hence, there are four or five successive plates, each one covering posteriorly the very anterior part of the next one (specimen NASU 25567a, Fig. 24A View FIG ): a small overlap area is seen at the very anterior edge of the isolated elements ( Fig. 24C, D View FIG ). The last dorsal plate of the tail, being higher and strongly arched indicates most likely a slender thickness of the tail, hence a distal position for this element ( Fig. 24F, I View FIG ).
The second and fourth postmedian dorsal plates show on their dorsal side a little scar, that is probably an attachment area for a – separately ossified – little dorsal spine (bigger for the last plate), however not found in the material.
Post-median ventral “scutes” (PMV.sc, Fig. 24A, J, K View FIG ). They covered the ventral side of the tail. Tubercles are finer than on the postmedian dorsal plates. The ventral series begins more posteriorly than the dorsal one, in order to let the cloacal pore (or at least the anus) free. The specimen NASU 25567a ( Fig. 24A View FIG ) shows a series of four plates, very low and slightly transversally curved. The posterior edge of each plate covers (externally) the anterior edge of the next one. The last plate is the smallest.
Four lateral scales of the tail (l.sc, Fig. 24A View FIG ). They, being bean-shaped, can be seen on the specimen ( Fig. 24A View FIG ) (8 mm high × 3 mm long). The concavity is forewardly directed. The reconstruction of the dorso- and ventrolateral scales in Kujdanowiaspis podolica (dl.lc, vl.lc, Fig. 16D View FIG ) is based on what Goujet observed in Sigaspis lepidophora ( Goujet 1973: fig. 3A).
Endoskeletal pectoral girdle
The endoskeletal pectoral girdle consists in an undivided and trifurcate scapulocoracoid, surrounded by the anterolateral, anterior ventrolateral, interolateral and spinal plates. The girdle can be divided into three parts: a coracoid and a prepectoral processes and an axillar area.
Coracoid process (p.cor, Fig. 22A). It is visible as an impression on specimen GGI 15-634 ( Fig. 22A), as well as the neurovascularization
c 3 f. dl c1
A?c4
AVL
(both small foramina and short grooves) that occurred between the anterior ventrolateral and spinal plates. The process was located against the ventrolateral and ventral parts of the anterior ventrolateral plate.
the scapulocoracoid, NHM P 18226: A, internal view ; B, external
Prepectoral process (p.pr.pec, Fig. 22A,C).It consisted in a cartilaginous fi lling of the hollow spinal plate (see GGI 15 634, RAS 2172/2). As the cartilage did not extend to the apex of the plate,the apex appears always rounder than the compacted rest of the plate.
Axillary area ( Fig. 25A, B View FIG ). It is visible on specimen NHM P 18226, already figured by Goujet (1984: fig. 71C, D, pl. 13, fig. 1). Two natural casts show respectively the internal side of the plastron (with the scapulocoracoid impression surrounded by the right interolateral plate), and both the internal and external structures of the axillary area (surrounded by the right anterior ventrolateral, anterolateral and spinal plates).
The relief is quite complex. The articular crest (cr.art, Fig. 25B View FIG ) is not located on any larger base, as occurs in Dicksonosteus arcticus ( Goujet 1984) . It is 5 mm long. A shallow longitudinal depression indicates that the articular surface of this crest was not ossified perichondrally. This articular crest is slightly bilobate (the anterior part is larger than the posterior), like in Dicksonosteus . Hence Goujet (1984) believed that two basalia could attach this crest, as is the case in Pseudopetalichthys Moy-Thomas, 1939 ( Gross 1962: figs 7, 8A; Stensiö 1969: fig. 246, p. 609), or maybe three as in selachians (Bendix-Almgreen 1975). In the latter case, the median basalia (mesopterygium) would have been reduced compared with anterior (anterior propterygial) and posterior (posterior mesopterygial) elements. On the contrary, a large cartilaginous disc attached on the biggest lobe of the crest and maybe a smallest element on its shortest part would be a better interpretation of this pattern (see Goujet 2001 and further).
Dorsally and ventrally to the crest, two longitudinal depressions can be seen, on which are attached the levator (s.m.add.pd, Fig. 25B View FIG ) and depressor muscles of the pectoral fin (s.m.add.pv, Fig. 25B View FIG ). It is noteworthy that in Dicksonosteus , these depressions are situated on the base of the articular crest. The pectoral fins unlikely had any important role in the locomotion of the fish; it is more likely that they had a stabilizating function, some supplementar “flying surface”, and/or were used as plane ailerons and flats in order to swim up and down in the water.
Four foramina are visible both on the external and internal sides.
The anterior foramen (c1) is located just posteriorly to the lengthened anterior pit (f.av), whereas in Dicksonosteus this pit is shorter and deeper, and the c1 foramen is located in its middle. From this foramen runs a short anterolaterally directed canal (external view, Fig. 25B View FIG ).
The medial foramen (c2) is located just behind the articular crest, and has the same size as c1. A short posteriorly and slightly dorsally directed canal runs from this foramen. This aperture (c2) is positionned below the opening of the c3 foramen. As is the case in Dicksonosteus , the medial foramen is located anteromedially in the posterior pit. This pit is divided into a dorsal and a ventral parts, demilited by a slight longitudinal crest (visible on the internal side).
The posterior foramen (c3) is situated above and behind the articular crest, in the dorsalmost part of the posterior pit. It is slightly smaller than the other two foramina (in the internal side of the axillary area of Dicksonosteus , c3 is the biggest foramen, on the external side it is about the same size as c1; Goujet 1984: figs 69, 70).
The excellent preservation of this specimen shows that, as assessed by Goujet (1984) observing Dicksonosteus , the first two foramina are endoskeletal, and the third one is rather a notch in the posterodorsal edge of the endoskeleton than a real pore.
Anteriorly to the anterior foramen c1 is located the anterior pit, which is hollowed out by a shallow groove (probably a muscle insertion, c.cut.a, Fig. 25B View FIG ).
The complex formed by the articular crest, the foramina and the anterior and posterior pits is surrounded by a very thin and shallow groove (dorsally s.m.ds, and ventrally s.m.vs, Fig. 25B View FIG ). This groove corresponds to the junction between the dermal exoskeletal bone and the perichondral bone. Goujet (1984) thought that it may also have an attachment area for the superficial muscular undercutaneous muscles, disposed as a dorsal sheet.
The fourth foramen (?c4, Fig. 25A View FIG ), without any identified homologous structure in Dicksonosteus , opens posteriorly to (and in the same plane as) the medial foramen (c2). The corresponding canal is posterodorsally directed. The foramen is not visible on the internal side; it would be located at the level of the posterior pit, according to Goujet (1984) (f.dl, Fig. 25 View FIG ).
A minor foramen opens in the distal angle of the anterior fossa (c.cut.a) on the internal side. Another opens in the distal angle of the posterior fossa, but its shape is blurred (c.cut.p, Fig. 25B View FIG ). These structures can be seen at the same place in Dicksonosteus , and were interpreted by Goujet (1984); they would consist in canalicles of the anterior and posterior cutaneous nerves of the scapulocoracoid. The anterior canal should come from the same group as the vessels and nerves from foramen c1. A similar condition occurs in Dicksonosteus . The posterior canal, as in Dicksonosteus , should have been linked to the internal side of the armour via an oblique canal, and probably let the passage for some vessels and/or nerves coming from the abdominal area and going to the natatory lobe of the tegument ( Goujet 1984).
As assessed by Goujet (1984), one can propose an analogy rather than a homology between these three main canals and the encountered disposition in extant fishes(selachians: Squatina Linnaeus, 1758 , Marples 1936; actinopterygians: see Jessen 1972) and other placoderms illustrated by Stensiö (1944, 1959, 1969).
Suprasynarcual: a new element of the vertebral column
The elements of the vertebral column were cartilaginous and hence are very rare in the fossil record.One isolated specimen was found in the material. It is a new element called“suprasynarcual” (NHRM P 8479, Fig.27 View FIG ). Indeed, it does not look like the synarcual of Dicksonosteus arcticus ( Goujet 1984: fig. 73, p. 150) or of any other placoderm.
The suprasynarcual described herein is an unpaired medial chondrified element that was stuck against the internal side of the median dorsal plate of Kujdanowiaspis (the latter being very easy to recognize because of its curvature). So, the suprasynarcual is visible in its ventral side only. The dorsal side of the suprasynarcual likely moulded the visceral side of the median dorsal plate.
The suprasynarcual looks like a concave reversed water-drop.It shows an eroded medial crest(cr, Figs 27 View FIG ; 28 View FIG ), apart of which three pairs of foramina can be seen (the left most posterior one is slighlty laterally displaced compared to the others). These were probably vascular foramina, rather than neural ones.
A reconstruction of the suprasynarcual in the armour is attempted as a hypothetical transversal cut in the anterior part of the thoracic armour ( Fig. 28 View FIG ). This shows that the suprasynarcual is placed higher than the occipital condyles of the neurocranium.Possibly its ventral crest contacted the neural arch of the synarcual (an.syn, Fig. 28 View FIG ). So the foramina of the suprasynarcual were probably not neural structures, but rather nutritive foramina for the cartilage.
As the overlapping areas of the anterior dorsolateral plates are quite important, it is likely to consider that the lateral edges of the suprasynarcual contacted the mesiodorsal borders of these overlapping areas. This assemblage probably assessed a better cohesion of the thoracic armour, as it reinforced the anterior part of the vertebral column rigidity.
Goujet (1984) thinks that the synarcual of Dicksonosteus arcticus was attached to the anterior crest of the visceral side of the median dorsal plate (ra, Goujet 1984:fig.59B, p. 130).These two elements were not fused, but more probably attached by ligaments and/ or muscles.An anterior crest is also visible on the median dorsal plate of Arctolepis decipiens ( Goujet 1984: pl.19,fig.6). It is unlikely that these two phlyctaeniids genera also possessed a suprasynarcual element. The presence of such an element in Kujdanowiaspis podolica would more likely be an answer to the height of the median dorsal plate.
The presence of this suprasynarcual element and its function in maintaining the vertebral column reminds the ventral keel of the brachythoracids. But the suprasynarcual of Kujdanowiaspis is chondrified, whereas the brachythoracid ventral keel is clearly ossified and consists in a true process of the median dorsal plate, not as an annex ossification. But if these two structures are homologous, it is possible that during evolution the suprasynarcual fused with the median dorsal plate in brachythoracids. Nevertheless, this hypothesis is still impossible to test.
Ornamentation of Kujdanowiaspis podolica ( Brotzen, 1934)
Tubercles are generally coarser on the dorsal and lateral sides rather than the ventral side of the armour. This differentiation is maybe linked to a sub-benthic life of the fish. Nevertheless, no worn surface is visible.
Tubercles are also finer beneath areas loosely covered by another (e.g., the submarginal over the postmarginal plate).
Another function of the ornamentation consists in a passive defence. The plates of the armour are generally robust. Moreover, the long spinal plates may play a dissuasive role, virtually increasing the volume of the animal (cf. Janvier 1985 a: 225, discussion about the boreaspidid osteostracans). As well, the mesial denticles of the spinal and post median dorsal plates are as numerous spines.
The whole armour could also consist in a phosphat reserve for the muscular activity of the fish. This would imply that tubercles did not emerge from the superficial layers of the dermis.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Kujdanowiaspis podolica ( Brotzen, 1934 )
| Dupret, Vincent 2010 |
Phlyctaenaspis podolica
| BROTZEN F. 1934: 114 |
Phlyctaenaspis extensa
| BROTZEN F. 1934: 116 |
Phlyctaenaspis rectiformis
| BROTZEN F. 1934: 117 |
Acanthaspis prominens
| BROTZEN F. 1934: 119 |
Acanthaspis angusta
| BROTZEN F. 1934: 121 |
| BROTZEN F. 1934: 120 |