Geodia papyracea ( Hechtel, 1965 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.191088 |
|
DOI |
https://doi.org/10.5281/zenodo.5689936 |
|
persistent identifier |
https://treatment.plazi.org/id/03FD6D27-AA55-323E-FF4B-2F6CBC51EC4D |
|
treatment provided by |
Plazi |
|
scientific name |
Geodia papyracea ( Hechtel, 1965 ) |
| status |
|
Geodia papyracea ( Hechtel, 1965)
( Figure 16 View FIGURE 16 )
Synonyms.
Geodia (Cydonium) papyracea Hechtel, 1965: 71 , text-fig. 13, pl. VIII, figs.1–2. Geodia papyracea Hechtel, 1965 : Alcolado 1981, p. 38.
Holotype. YPM 5045, mangrove boat channel, Port Royal, Jamaica.
Material. UMPCW 921, Solarte Island, on a mangrove root, 0.5–1 m depth.
Additional material examined. G. papyracea , YPM 5045, holotype, mangrove boat channel, Port Royal, Jamaica; YPM 5309, 5311, paratypes, mangrove boat channel, Port Royal, Jamaica; USNM 42662, mangrove, Twin Cays, Belize.
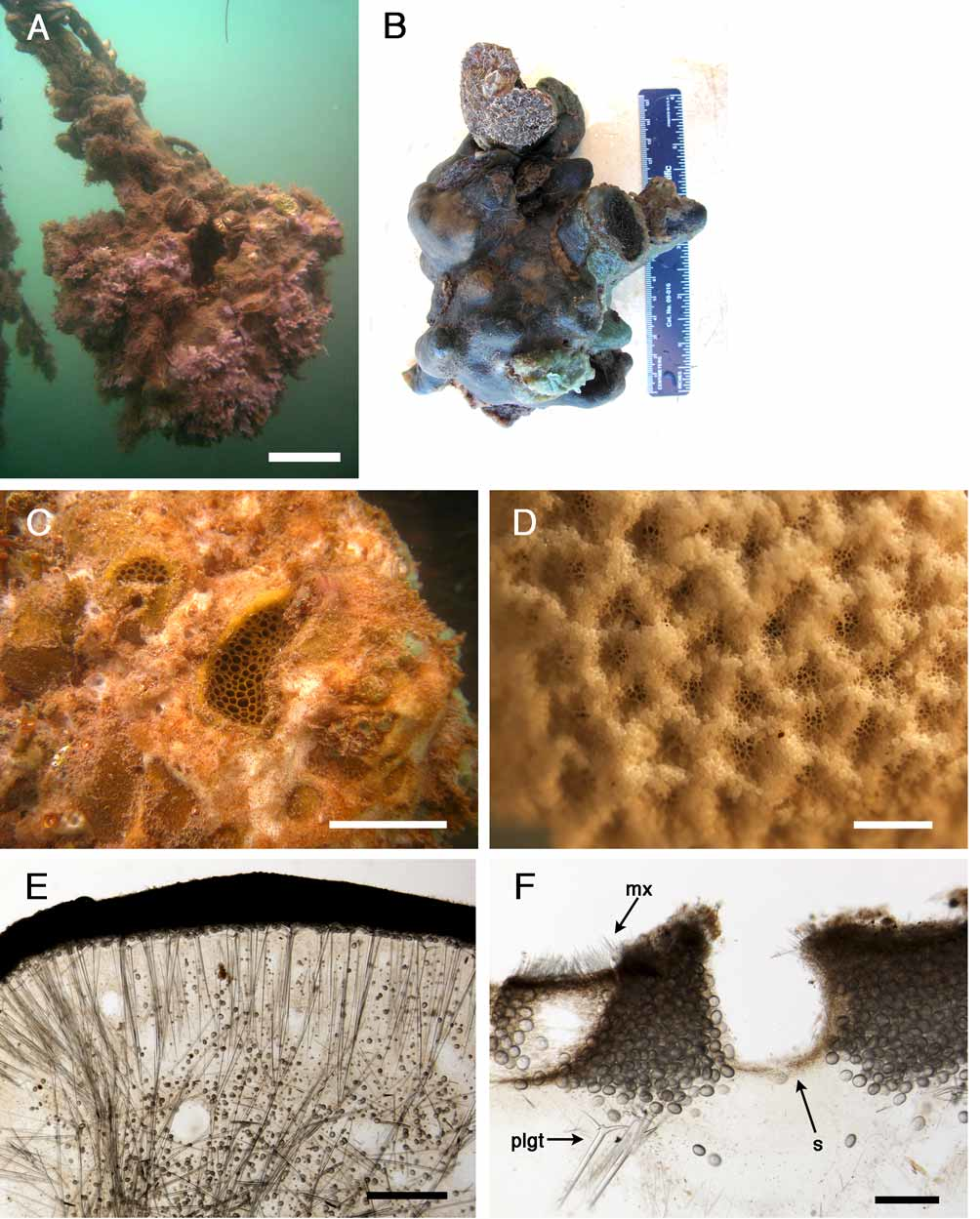
Outer morphology ( Fig. 16 View FIGURE 16 A). Whitish massive sponge with green tinges when alive. It is ca 6x 12 cm. The sub-sample we have is whitish in alcohol; the cortex being lighter than the choanosome. Oscules are uniporal, each with a sphincter and grouped in a sub–circular, slightly depressed plate. The oscule plate can be dark green. Pores are cribriporal and widely distributed over the entire surface. Each cribriporal plate has a diameter of 1 mm.
Skeleton ( Fig. 16 View FIGURE 16 B). The cortex is 480–840 µm thick and composed of a thin ectocortex of strongylasters (0–72 µm) and a thicker endocortex made of sterrasters (480–840 µm). The cortex is easily torn and detachable from the choanosome. Oxeas I and plagiotriaenes are positioned under the cortex, more or less radially. The plagiotrianes usually cross the cortex so that the cladomes end up in the ectocortex or at the surface of the cortex. The radial arrangement of megascleres is less obvious 1 mm under the cortex. Oxyasters I, oxyasters II and strongylasters can be found in the whole choanosome, as well as developing sterrasters. Oxeas II, smaller than oxeas I, are only found in the choanosome.
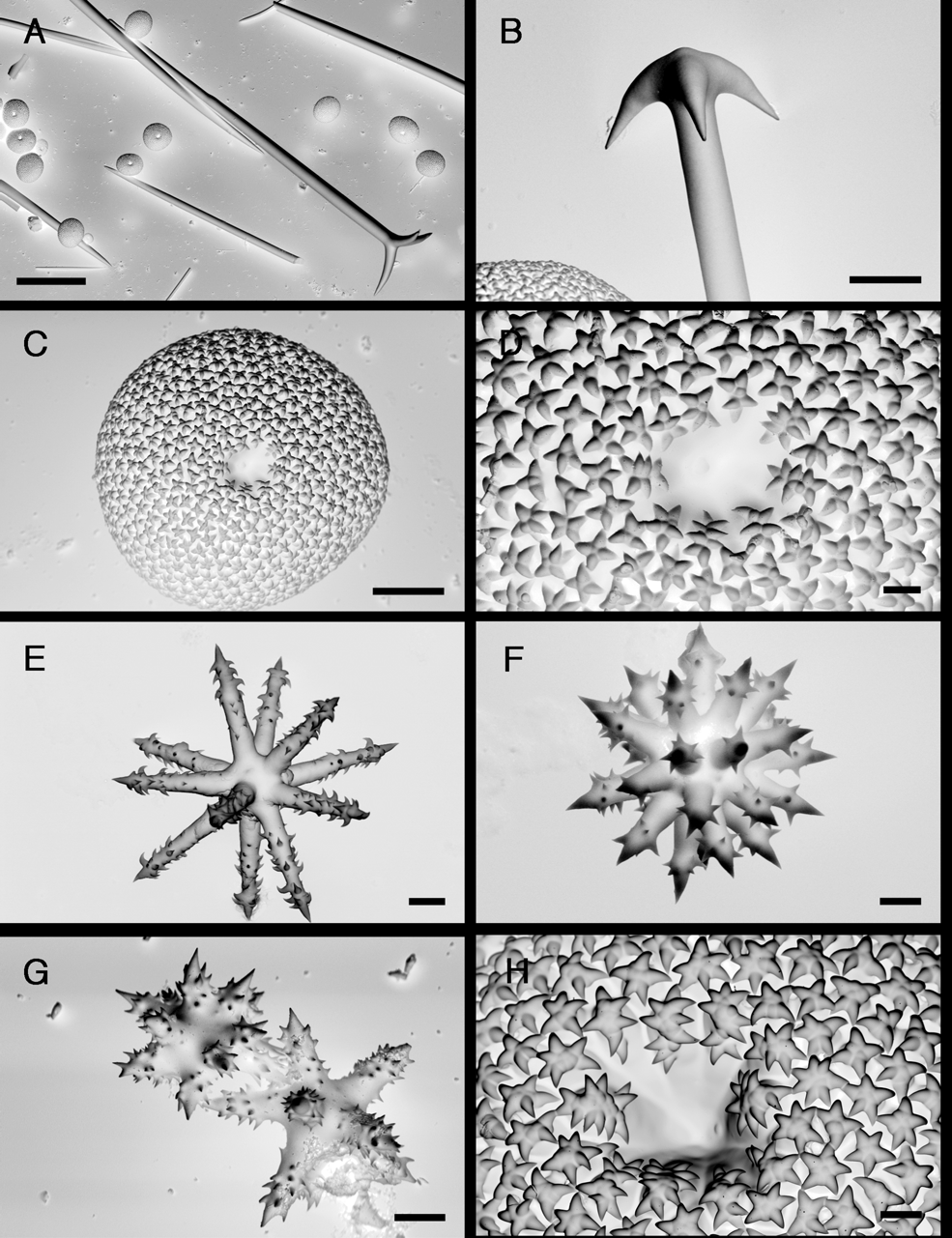
Spicules ( Fig. 16 View FIGURE 16 C–G). Megascleres: (a) oxeas I, stout, straight or slightly bent, length: 651– 1039 –1248 µm; width: 7– 24.3 –29 µm. (b) oxeas II, rare, sometimes with strongyle ends, usually straight, length: 95– 124.3 –244 µm; width: 1– 2 –3 µm (N=14). (c) plagiotriaenes, rhabdome length: 641– 962.7 –1080; rhabdome width: 13– 24.6 –34 µm; clad length: 57– 97.1 –132 µm. (d) anatriaenes, rare, rhabdome length: 354– 633.2 –885 µm (N=5); rhabdome width: 2.5– 3.2 –4 µm (N=8); clad length: 5– 13.8 –27 µm (N=8). (e) mesoprotriaenes, very rare, rhabdome length: 632 µm (N=1); rhabdome width: 3 µm (N=1); clad length: 9–16 µm (N=2).
Microscleres: (f) sterrasters ( Fig. 16 View FIGURE 16 C–D), subglobular, with warty 4–7 branched rosettes at their surface (diameter: ca 4 µm), length: 65– 71.6 –77 µm; width: 63– 70.3 –75 µm; thickness: 50– 55.9 –61 µm. (g) acanthoxyasters I ( Fig. 16 View FIGURE 16 E), 9–12 thin actines, center more or less developed, diameter: 22– 27.5 –36 µm. (h) acanthoxyasters II ( Fig. 16 View FIGURE 16 F), with large centrum and thicker actines than acanthoxyasters I, 9–17 actines, less common than acanthoxyasters I. diameter: 14– 23 –29 µm. (i) acanthostrongylasters ( Fig. 16 View FIGURE 16 G), 14–20 actines, diameter: 3– 4.9 –8.8 µm.
Habitat in the Bocas del Toro region. On mangrove roots, 0.5–1.5 m depth.
Distribution. Cuba ( Alcolado 2002); Belize ( Rützler 1988; Rützler et al. 2000); Panama (Díaz 2005); Jamaica ( Hechtel 1965); Colombia ( Wintermann-Kilian & Kilian 1984); Curaçao (van Soest 1981); Brazil ( Burton 1940; da Silva et al. 2004; Cedro et al. 2007).
Remarks and discussion. The fragile and easily peeled cortex of G. papyracea might be due to the peculiar disposition of the triaenes, with the cladomes not supporting the cortex as in most Geodia species. The ectocortex of the Bocas del Toro specimen was much thinner (0–72 µm) than in the Belize sample (120–325 µm) and the holotype / paratypes (93–232 µm thick). Conversely, the sterrastreal layer in the Bocas del Toro specimens was thicker (480–840 µm) than in the Belize sample (93–279 µm) and the holotype / paratypes (186–418 µm). This is partly a consequence of the larger sterrasters in comparison with the type material and previous G. papyracea records (da Silva et al. 2004). We also noted that the surface of the Bocas del Toro specimen was less folded, the parchment–like appearance characteristic of this species was therefore not as obvious as in the samples from Jamaica and Belize. The rest of our measurements were in accordance with the species description. This species was very common in Solarte lagoon in 2005 and 2006 (M. C. Díaz, personal observation). We were not able to find it anymore in 2007 and 2008 as it appeared to have been replaced by the sympatric species Geodia gibberosa .
In the field, G. papyracea can be mistaken with the previously described G. gibberosa since they are both found living on mangrove roots and can even be found growing on one another ( Hechtel 1965). Again, gross morphology is here important: G. papyracea has very poor relief and a fragile cortex while G. gibberosa has characteristic lobes and a tough and thicker cortex. G. gibberosa seems to be more overgrown than G. papyracea . In thick sections, the skeleton was more radially organized in G. gibberosa ( Fig. 14 View FIGURE 14 E), with plagiotriaenes supporting the cortex and not crossing it like in G. papyracea ( Fig. 16 View FIGURE 16 B). This probably accounts for the firm cortex in the former. Then, G. gibberosa has larger oxeas and plagiotriaenes; the sterrasters are also a bit larger and more oval shaped. SEM pictures of asters revealed additional microstructural differences. When comparing the surface of sterrasters, the G. gibberosa rosettes ( Fig. 15 View FIGURE 15 D) had less rays than those of G. papyracea ( Fig. 16 View FIGURE 16 D). In G. gibberosa , rosettes also had a smaller center and no warts on the rays. Seemingly, the hilum of the sterrasters were different (the hilum is a circular depression, a remnant of where the nucleus of the sclerocyte used to be): smooth in G. gibberosa , warty in G. papyracea . These differences were confirmed by SEM observation of the holotypes’ sterrasters ( Figs. 15 View FIGURE 15 H, 16H). We also noticed that the actines of the acanthoxyasters III in G. gibberosa ( Fig. 15 View FIGURE 15 G) have a more prominent spine at their tip when compared with the acanthostrongylasters of G. papyracea ( Fig. 16 View FIGURE 16 G). Finally, these two species had significantly different Folmer COI sequences (60 bp. difference) and 28S partial sequence (63 bp. difference). A molecular phylogenetic study shows that they belong to two clearly seperated Geodia clades ( Cárdenas et al. 2009).
Although the holotype (YPM 5045) has been fixed and preserved in alcohol, the DNA we extracted from it was poorly preserved. Consequently, we were unable to amplify the Folmer COI fragment or a 28S sequence from the type material.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Geodia papyracea ( Hechtel, 1965 )
| Cárdenas, Paco, Menegola, Carla, Rapp, Hans Tore & Díaz, Maria Cristina 2009 |
Geodia (Cydonium) papyracea
| Hechtel 1965: 71 |