Erylus formosus Sollas, 1886
|
publication ID |
https://doi.org/ 10.5281/zenodo.191088 |
|
DOI |
https://doi.org/10.5281/zenodo.5689932 |
|
persistent identifier |
https://treatment.plazi.org/id/03FD6D27-AA52-3204-FF4B-2ADFBAD2E899 |
|
treatment provided by |
Plazi |
|
scientific name |
Erylus formosus Sollas, 1886 |
| status |
|
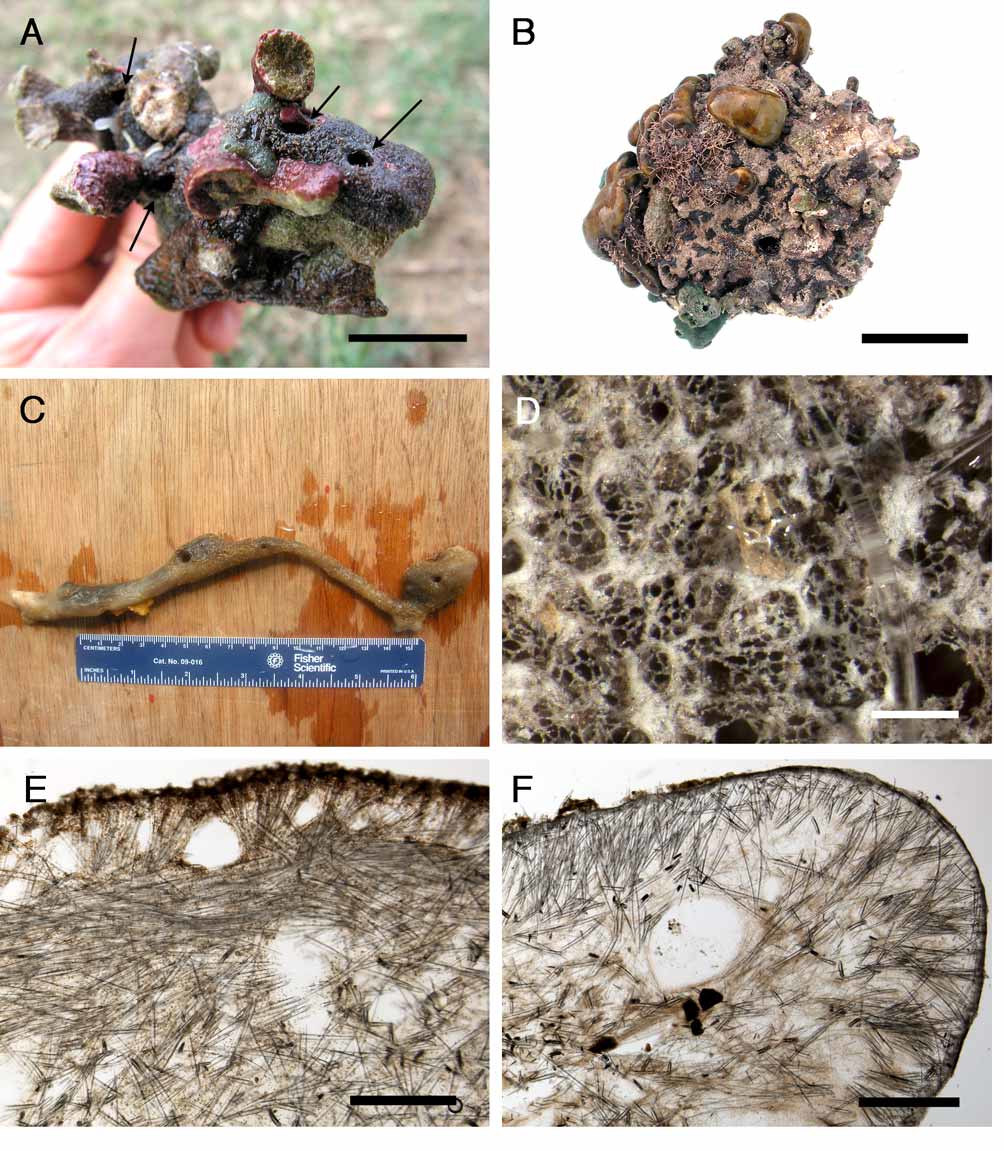
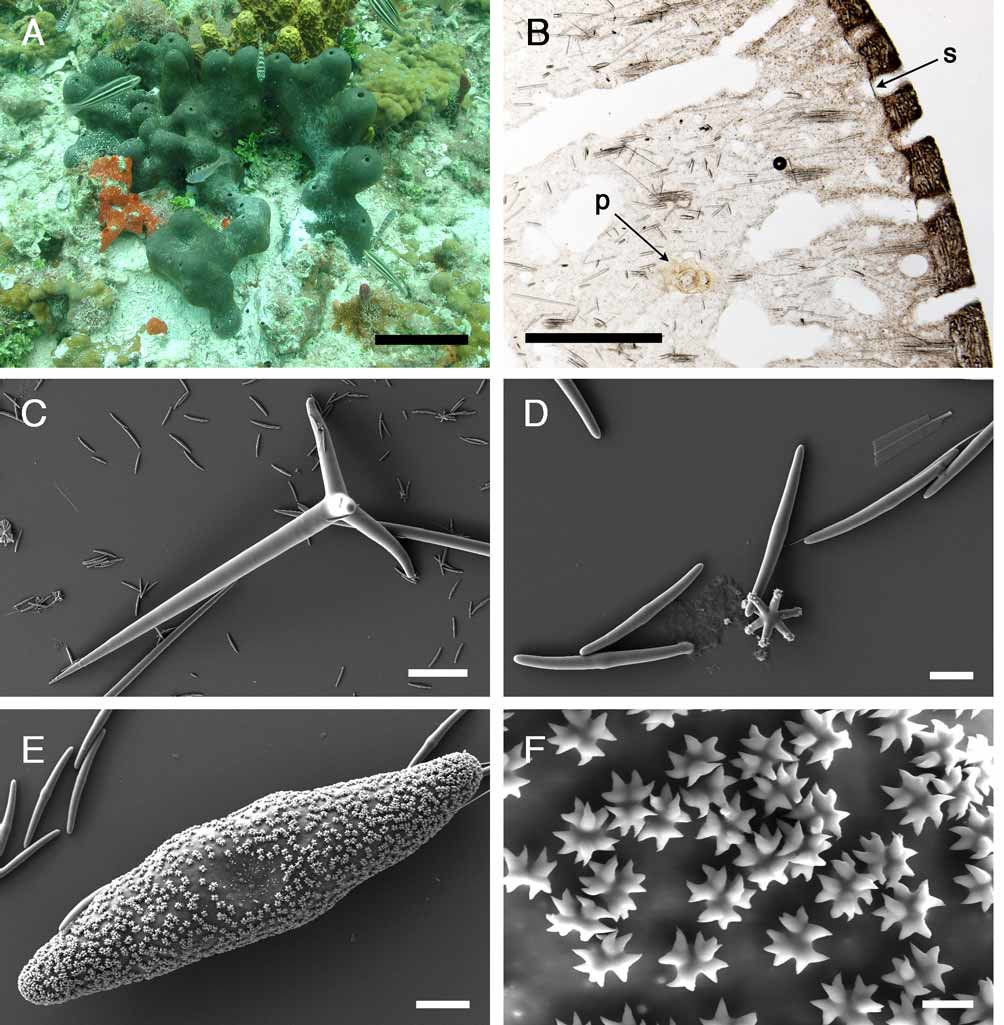
( Figure 13 View FIGURE 13 )
Synonyms.
Erylus formosus Sollas, 1886: 195 ; Sollas 1888: 209, pl. XXVIII. Erylus bahamensis Pulitzer-Finali, 1986: 78 , figs. 12–13 (new synonym).? Erylus clavatus Pulitzer-Finali, 1986: 80 , figs. 14–15.
Holotype. BMNH 1889.1.1.77, off Bahia, Brazil, 12–36 m (not seen).
Material. ZMBN 81644–81645, Adriana’s Reef, Bocas del Toro, 3 m depth.
Additional material examined. Erylus bahamensis , ZMBN 82978, Little San Salvador Island, Bahamas, det. P. Cárdenas; ZMBN 82979, Sweetings Cay, Grand Bahama Island, Bahamas, det. P. Cárdenas. Erylus formosus , MNHN DNBE –997, off Brazil, 7°29’S, 34°30’ W, 45 m, Calypso expedition; ZMBN 81781, Cat Cay, Bimini Island, Bahamas, det. P. Cárdenas.
Outer morphology ( Fig. 13 View FIGURE 13 A). Massive, lobate, 15 to 30 cm long, dark–brown sponge. Choanosome is whitish and dense. Cortex becomes blackish in alcohol while the choanosome remains whitish. Surface is very smooth, no epibionts were observed. Uniporal oscules (ca 3 mm) are placed at the top of vertical lobes. Each oscule leads into a cloaca in which exhalant canals flow in; no sphincters could be seen in these openings. Uniporal pores (ca 0.15 mm) are evenly distributed on the whole surface of the sponge; each pore has a sphincter ( Fig. 13 View FIGURE 13 B).
Skeleton ( Fig. 13 View FIGURE 13 B). The cortex (168–336 µm thick) is composed of a very poorly developed ectocortex of microrhabds and a thick endocortex of aspidasters, tangentially arranged. Orthotriaenes and oxeas under the cortex are more or less radially arranged, in small bundles; the cladomes of the orthotriaenes are juxtaposed to the endocortex. Under this peripheral arrangement (1 mm thick), bundles of oxeas tend to be paratangential. Acanthotylasters are present in the choanosome, and in the walls of canals. Microrhabds are present in the choanosome too. Dark granular cells (11–14 µm) are present from the cortex to the choanosome although their density decreases under the peripheral layer.
Spicules (measurements of ZMBN 81645) ( Fig. 13 View FIGURE 13 C–F). Megascleres: (a) oxeas, stout, straight or slightly bent, sometimes modified to styles, length: 780– 943.8 –1118 µm; width: 20– 24.2 –30 µm. (b) orthotriaenes to plagiotriaenes ( Fig. 13 View FIGURE 13 C), rhabdome with an oxeote end, rhabdome length: 286– 577.2 –728 µm; rhabdome width: 20– 31 –40 µm; clad length: 150– 197.3 –260 µm. Microscleres: (c) aspidasters ( Fig. 13 View FIGURE 13 E–F), irregular rod–shape, with enlarged center, smooth rosettes at their surface (diameter: ca. 2–3 µm), each one with 3–4 bifid or trifid rays, length: 180– 206.2 –226.1 µm; width: 37– 47.1 –55.9 µm; length/width: 4.1–5.9. (d) microrhabds ( Fig. 13 View FIGURE 13 D), smooth, usually centrotylote, straight or slightly bent, with strongylote ends, length: 34.6– 45.8 –55.9 µm; width: 2.7– 3.5 –5.3 µm. (e) oxyasters, 2–5 actines, irregular with no centrum, with slightly tylote end or not, diameter: 34.6– 47.3 –63.8 µm. (f) acanthotylasters ( Fig. 13 View FIGURE 13 D), 4–9 actines, diameter: 11– 17.4 –21.6 µm.
Habitat in the Bocas del Toro region. Patchy reef, 3–6 m depth.
Distribution. Florida Keys, USA (Chanas & Pawlik 1995; Kubanek et al. 2000); Bahamas ( Wiedenmayer 1977; Pulitzer-Finali 1986; Stead et al. 2000); Cuba ( Alcolado 2002); Puerto-Rico ( Carballeira & Negrón 1991); Jamaica (Lehnert & van Soest 1998); Virgin Islands (USNM 31489, 31579, 41227); Dominican Republic ( Pulitzer-Finali 1986); Curaçao (van Soest 1981); Mexico ( Lehnert 1993; Antonov et al. 2007); Belize (USNM 32377); Panama ( Collin et al. 2005; Díaz 2005); Venezuela ( Alvarez et al. 1991); Brazil ( Sollas 1888; Boury-Esnault 1973; Volkmer-Ribeiro & de Moraes 1975; Solé Cava et al. 1981; Mothes et al. 1999)
Remarks and discussion. ZMBN 81644 fitted the description of E. bahamensis Pulitzer-Finali, 1986 while ZMBN 81645 fitted the description of E. formosus . Indeed, the single morphological difference between E. bahamensis and E. formosus is the absence of the largest category of asters in the former. Apart from this unique spicule difference, both specimens were identical, from the same locality and depth. Wiedenmeyer (1977) had noticed these single aster E. formosus but thought it was not a strong enough character to create a different species. However, a few years later, Pulitzer-Finali (1986) decided it was enough to create a different species. We think the largest asters can be more or less widespread in the choanosome, and may even be totally absent. Depending where the section or spicule preparation are made, they can be overlooked, hence records of single aster E. formosus ( Wiedenmayer 1977; Lehnert & van Soest 1998) and E. bahamensis ( Pulitzer-Finali 1986; Alcolado 2002). Mothes et al. (1999) suggested that E. bahamensis further differed from E. formosus by its aspidaster proportions (length/width). We reviewed this proportion in the literature and found a proportion of 4.5–6.5 for most E. formosus , including the holotype ( Sollas 1888; Volkmer-Ribeiro & de Moraes 1975; Mothes et al. 1999), 4.1–5.9 for the Bocas del Toro specimens and 2.3–7.1 for the holotype of E. bahamensis ( Pulitzer-Finali, 1986, Figure 13 View FIGURE 13 ). To conclude, having shown that (i) large asters can be missing or overlooked and that (ii) there is no clear difference in aspidaster proportion between the two species, we consider E. bahamensis to be a junior synonym of E. formosus . Interestingly, we noticed that a few specimens from Brazil are out of the length/width range found above, and possess unusually narrow aspidasters (width: 11–20 µm) and thus present an aspidaster proportion of respectively 12.5 and 11.5 (Boury- Esnault 1973; Mothes et al. 1999). However, after reexamination of one of these specimens (MNHN DNBE– 997), we could find no other character to distinguish it from E. formosus . Another Caribbean Erylus , E.
clavatus Pulitzer-Finali, 1986 from Jamaica, has two types of asters but an aspidaster proportion of 2.2–3.5, a bit lower than most E. formosus . We think that its club-shape and its slightly lower aspidaster proportions are not enough to consider E. clavatus a valid species. It is most likely a junior synonym of E. formosus , as earlier suggested (Lehnert & van Soest, 1998).
Few morphological differences were observed between our Bahamas and Bocas del Toro samples. The Bahamas samples had a thinner cortex (144–160 µm) with flatter aspidasters; also, their asters had thinner actines.
We noticed that the rosettes on the aspidasters of the Bocas del Toro specimens ( Fig. 13 View FIGURE 13 F) were more complex than the ones of a Brazilian E. formosus specimen (MCNPOR 3379) ( Mothes et al. 1999, Fig. 5 View FIGURE 5 F). More data is required to ascertain if the rosettes’ morphology is a relevant discriminating microstructure in Erylus species.
E. formosus was found on two sites in Bocas del Toro ( Table 1) and is relatively common in Adriana’s reef. Studies show that it harbors no photosynthetic symbionts ( Erwin & Thacker 2007). On the other hand, we found small 4–5 mm long Syllidae polychaetes (F. Pleijel, personal communication) living in the canals of our specimen ( Fig. 13 View FIGURE 13 B), as well as a copepod. The Bahamas specimens examined also harbored numerous polychaetes.
Unfortunately and despite substantial efforts (DNA washing notably), we were not able to obtain a molecular sequence for this species, even from our Bahamas specimens. It has been previously shown that this species is particularly rich in proteins, carbohydrates and lipids when compared to other reef sponge species (Chanas & Pawlik 1995). Triterpene glycosides, for example, are used by this species for chemical defense against fish predation, fouling and microbial attachment ( Kubanek et al. 2000; Kubanek et al. 2002). Some of these compounds may act as DNA contaminants and block the PCR reactions.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Erylus formosus Sollas, 1886
| Cárdenas, Paco, Menegola, Carla, Rapp, Hans Tore & Díaz, Maria Cristina 2009 |
Erylus formosus
| Pulitzer-Finali 1986: 78 |
| Pulitzer-Finali 1986: 80 |
| Sollas 1888: 209 |
| Sollas 1886: 195 |